Question Video: Selecting the Correction Equation for the Reversible Reaction of Hydrogen Chloride and Ammonia to Make Ammonium Chloride | Nagwa
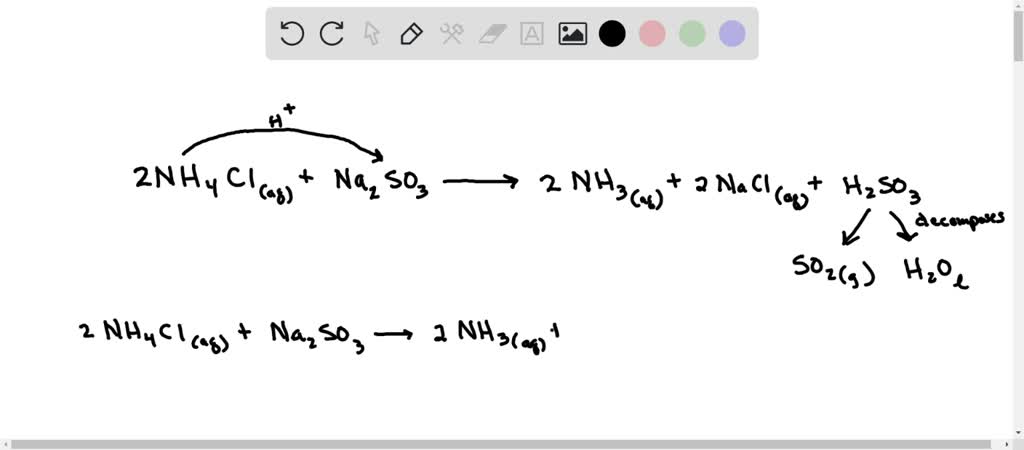
SOLVED: what's the acid base reaction for NH4Cl + Na2SO3 I keep putting in NH4^+ + SO3^2- –> NH3 + HSO3^- but it tells me its not an equilibrium

pH calculation of a buffer solution made from a weak base and its conjugate acid (salt form) - YouTube