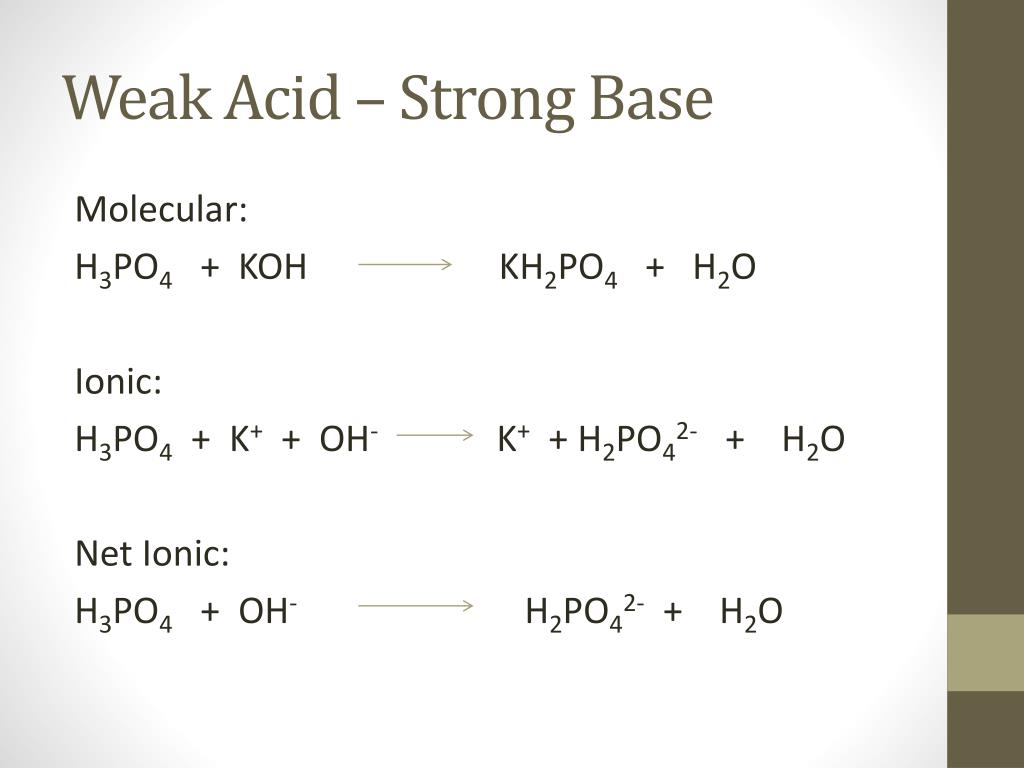
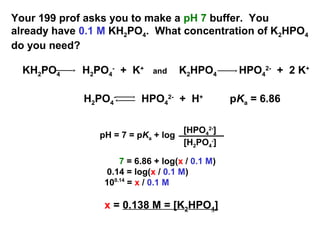
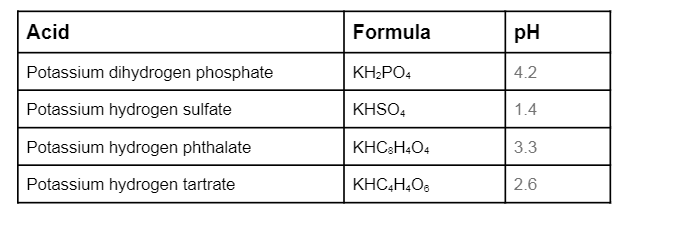
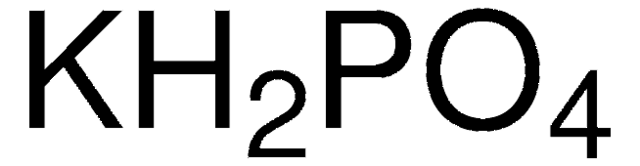14.87 | Calculate the pH of a buffer solution prepared from 0.155 mol of phosphoric acid, 0.250 mole - YouTube

Calculate the pH of a buffer solution obtained by dissolving 25.0 g of KH2PO4(s) and 38.0 g of Na2HPO4(s) in water and then diluting to 1.00 L. | Homework.Study.com

Phase Equilibrium for the Ternary System KH2PO4 + NaH2PO4 + H2O at 303.15 K | Journal of Chemical & Engineering Data

Effect of KH2PO4 concentration in the mobile phase on the retention... | Download Scientific Diagram

Table 1 from Natural Plant Extracts as Acid-Base Indicator and Determination of Their pKa Value | Semantic Scholar
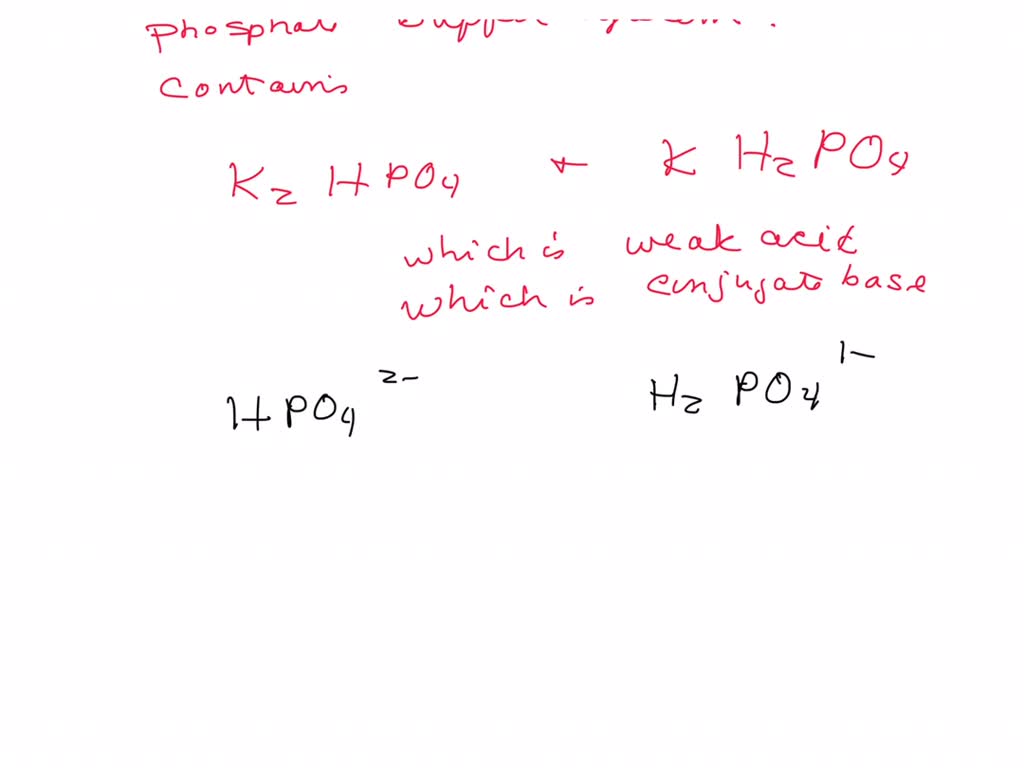
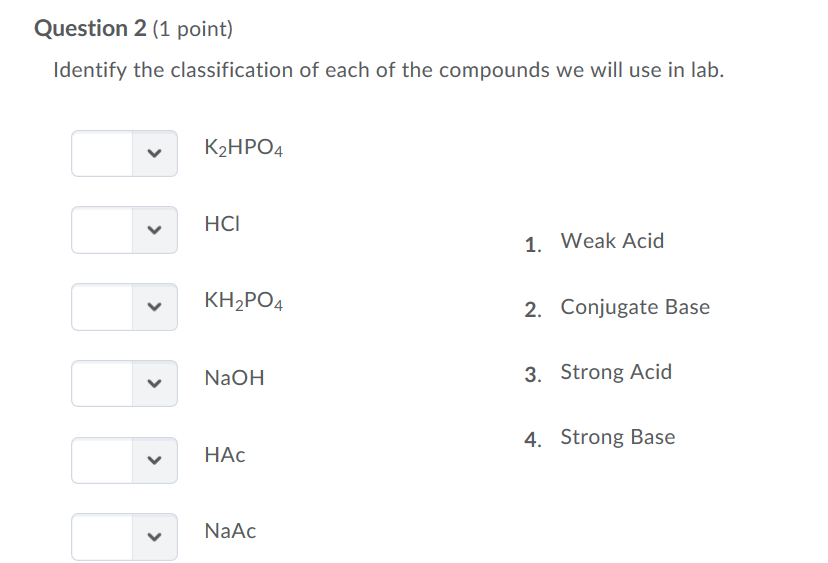
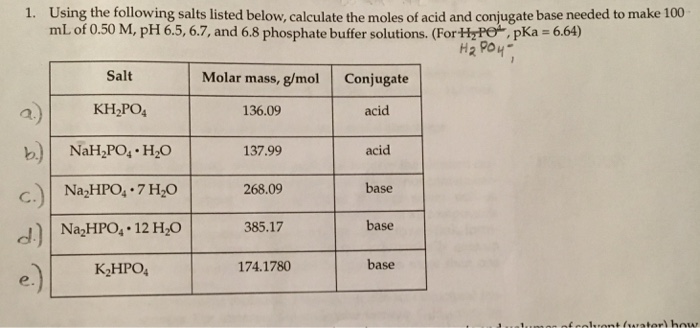
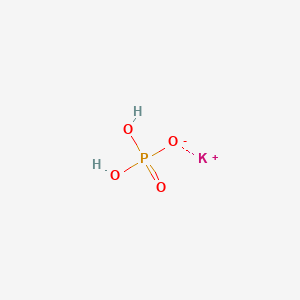
SOLVED: In the phosphate buffer system containing K2HPO4 and KH2PO4, what is the weak acid? What is its conjugate base?