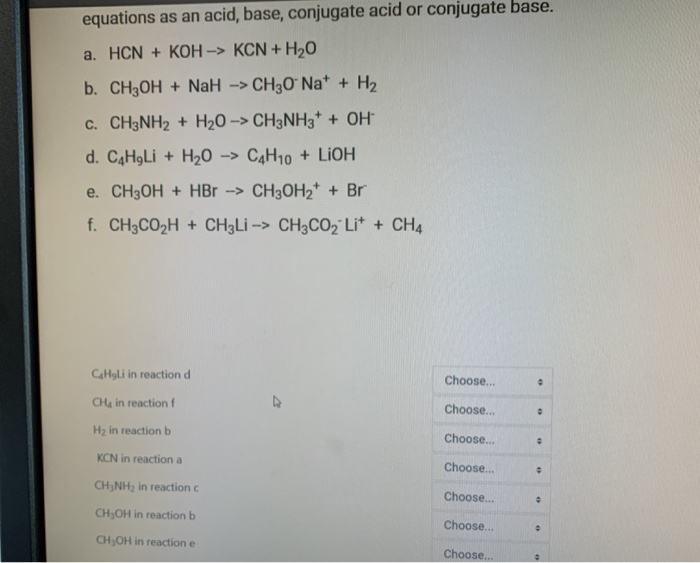
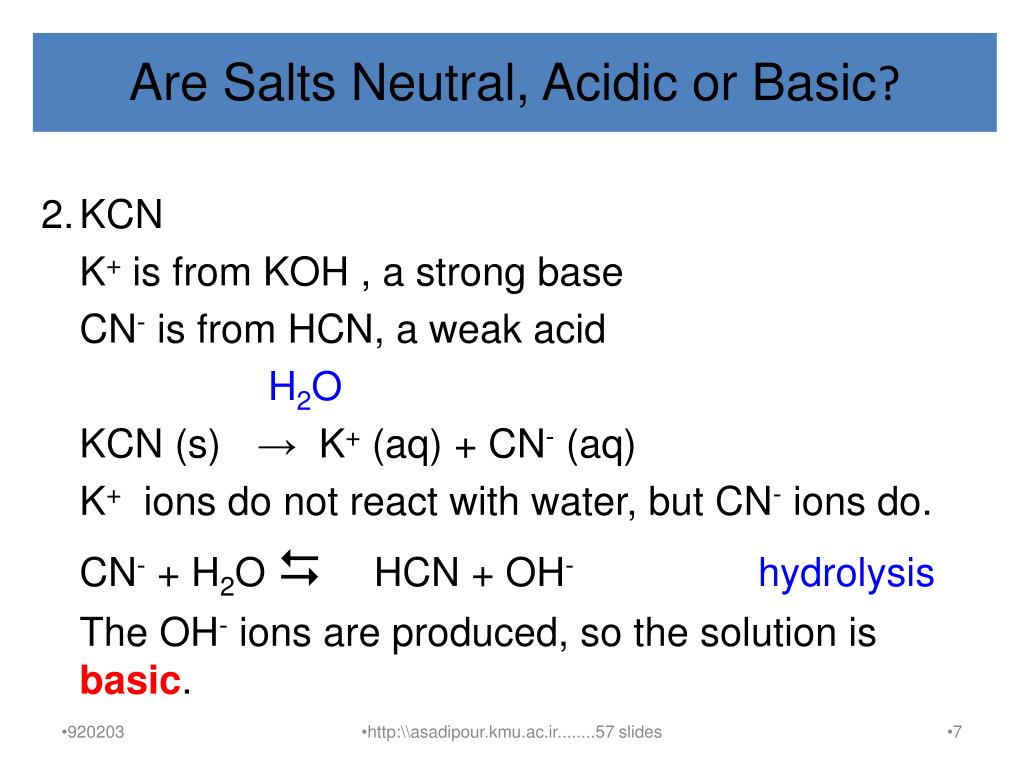
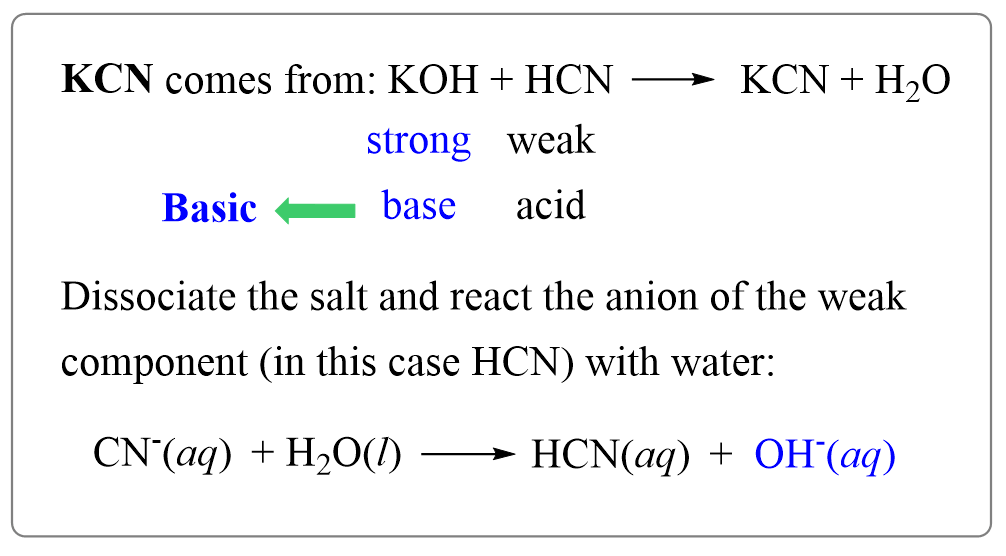
OneClass: Consider the following data on some weak acids and weak bases: acid base C0 name formula na...

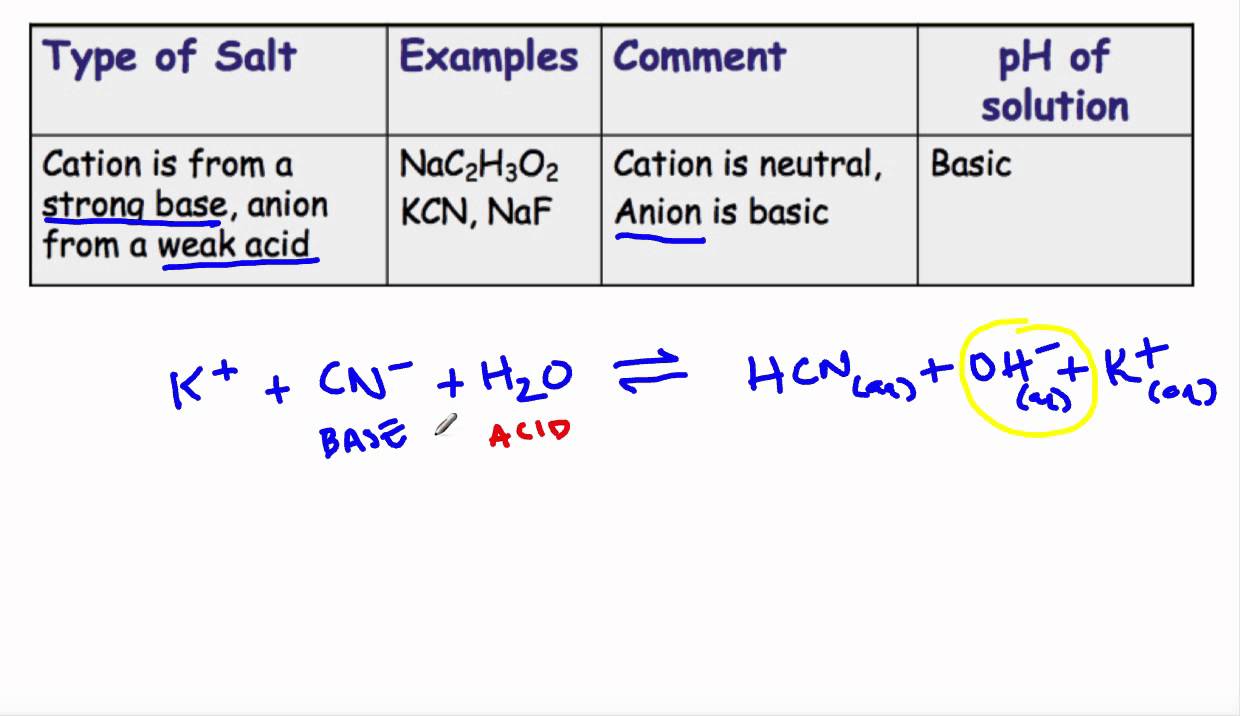
How to Determine if Salt is Acidic, Basic, or Neutral Example, Problem, Shortcut, Explained Question - YouTube
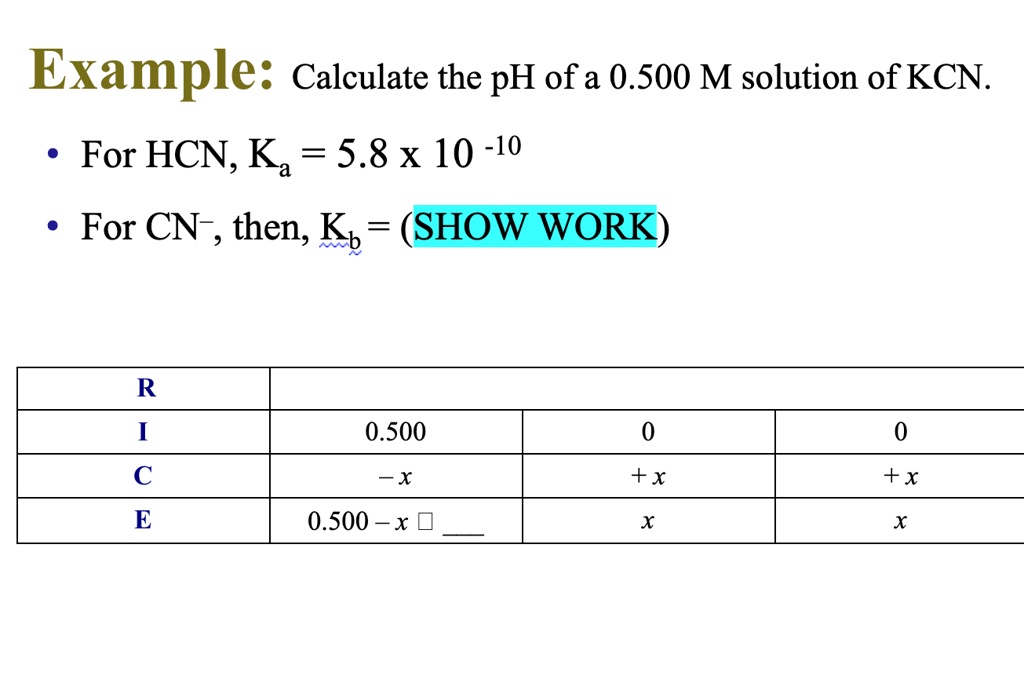
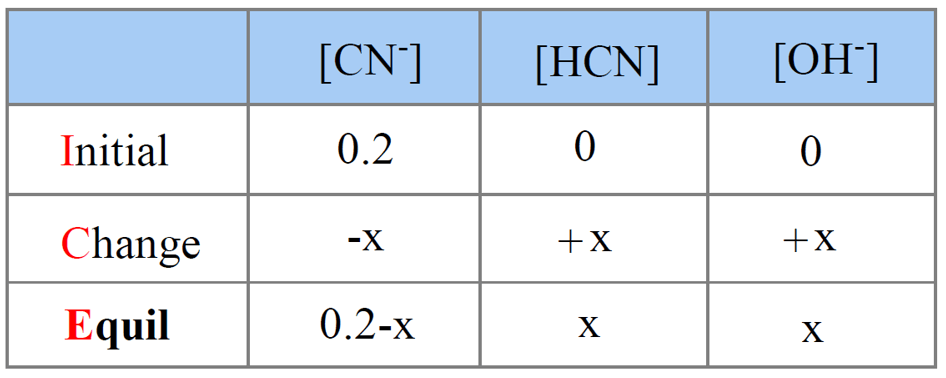
SOLVED: Example: Calculate the pH ofa 0.500 M solution of KCN For HCN, K = 5.8 x 10 -10 For CN , then, Kb (SHOW WORK) R 0.500 c E X 0.500 -x 0 X +X +X

SOLVED: You will find it useful to keep in mind that HCN is a weak acid acids: 0.1 mol of NaOH is added to 1.0 L ofa 0.5 MHCN solution. bases: other: