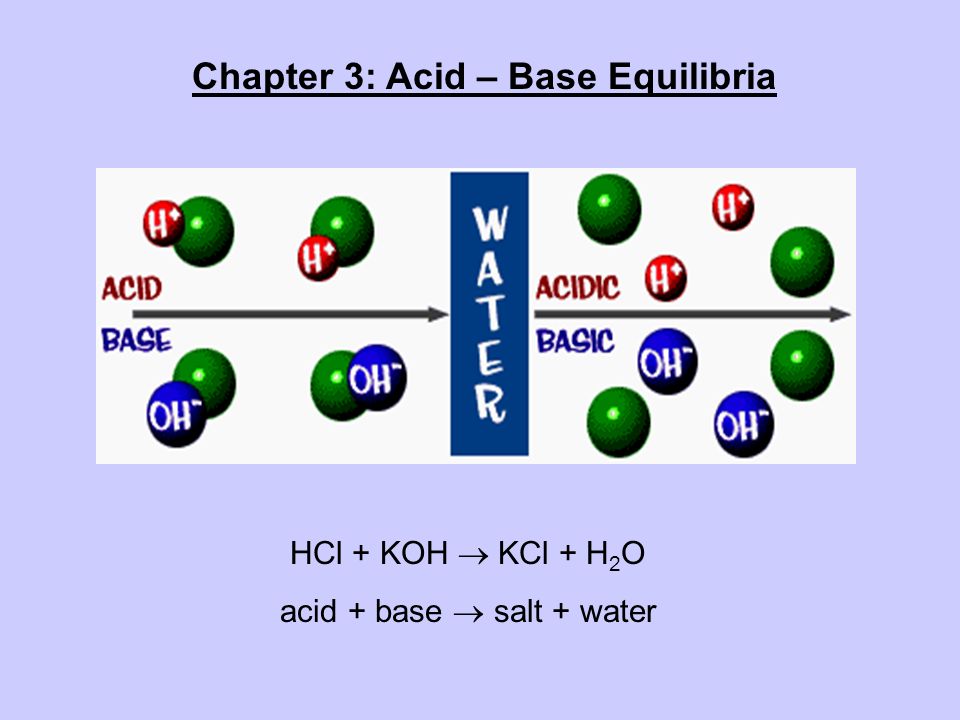
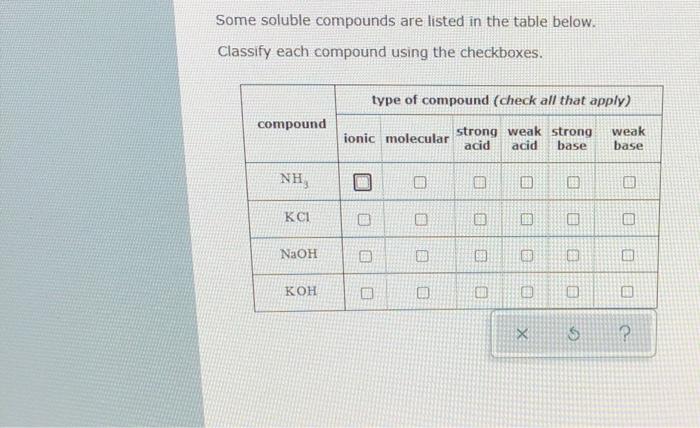
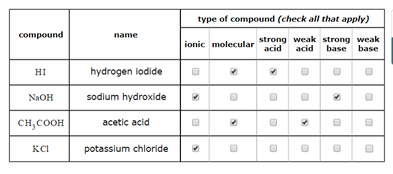
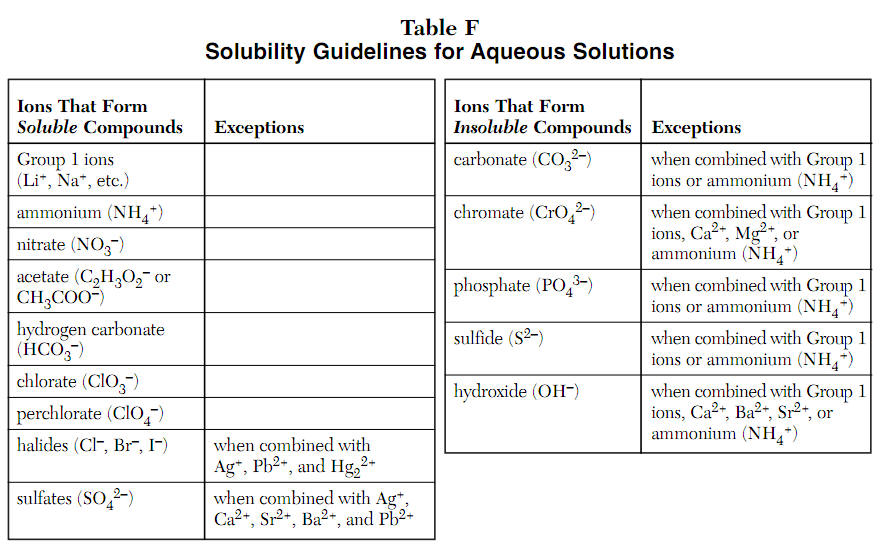
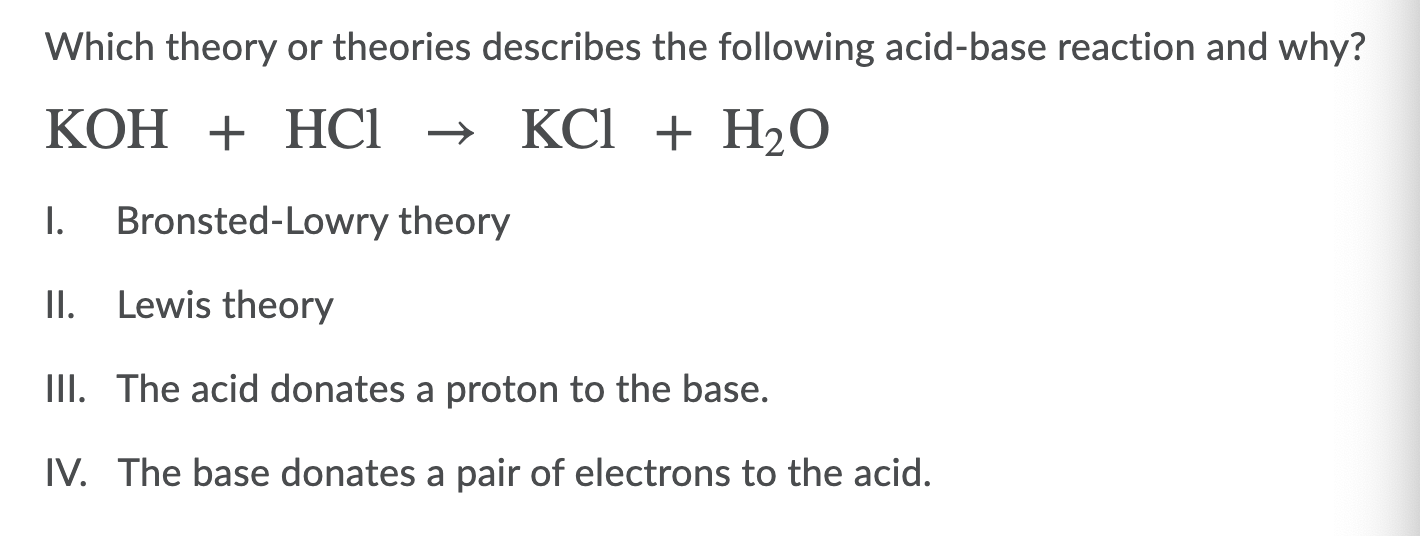
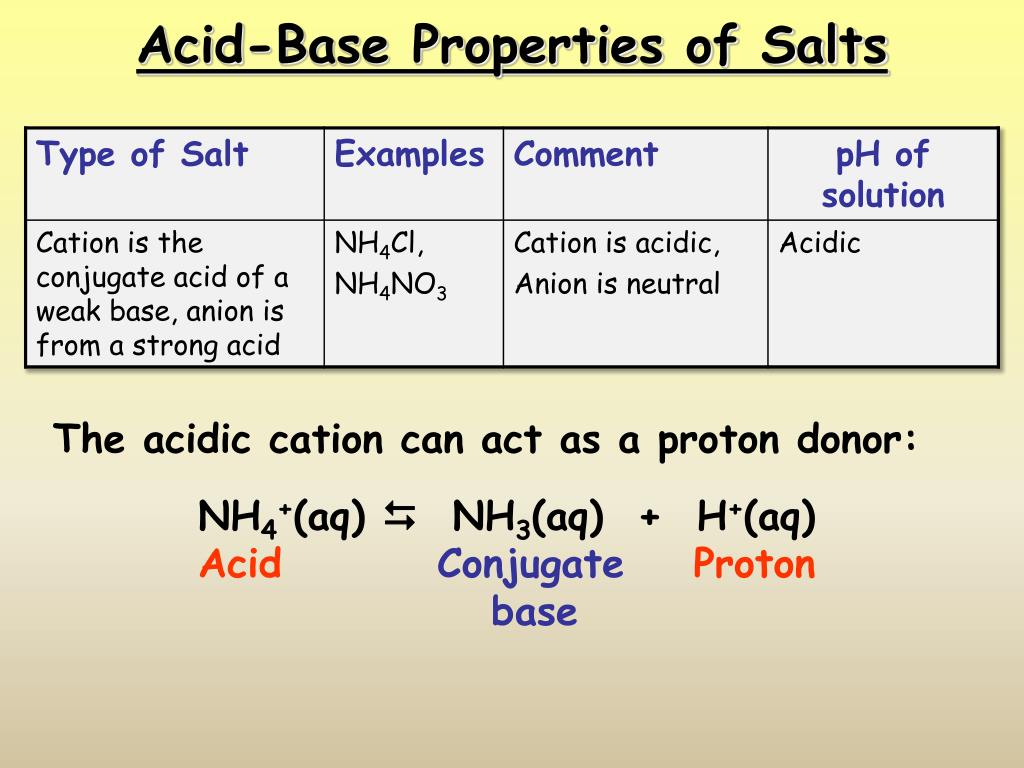
Acid and Base Chemistry. Some Properties of Acids þ Produce H + (as H 3 O + ) ions in water (the hydronium ion is a hydrogen ion attached to a water molecule) - ppt download

Acid and Base Production from 1.25 M NaCl, 0.2 M KCl, 0.008 M K 3 PO 4... | Download Scientific Diagram
Acid Base Titrations, Neutralization, Endpoint, Equivalence Point, Grade 11 Chemistry Power Point | Teaching Resources
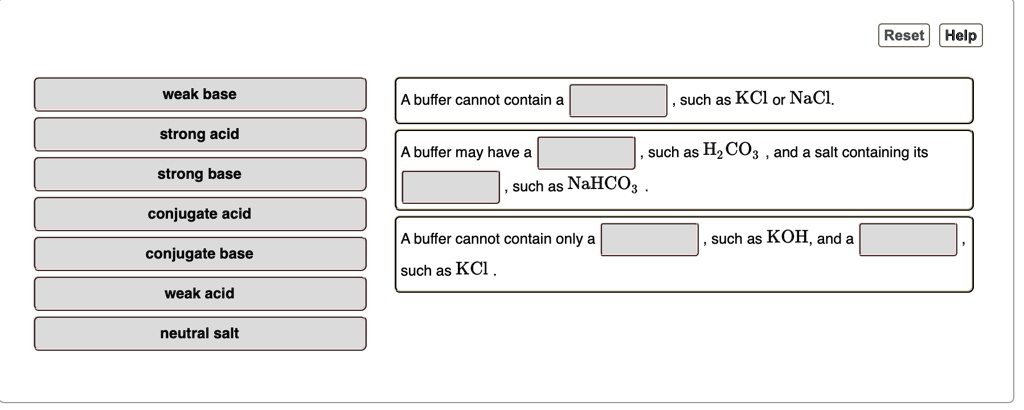
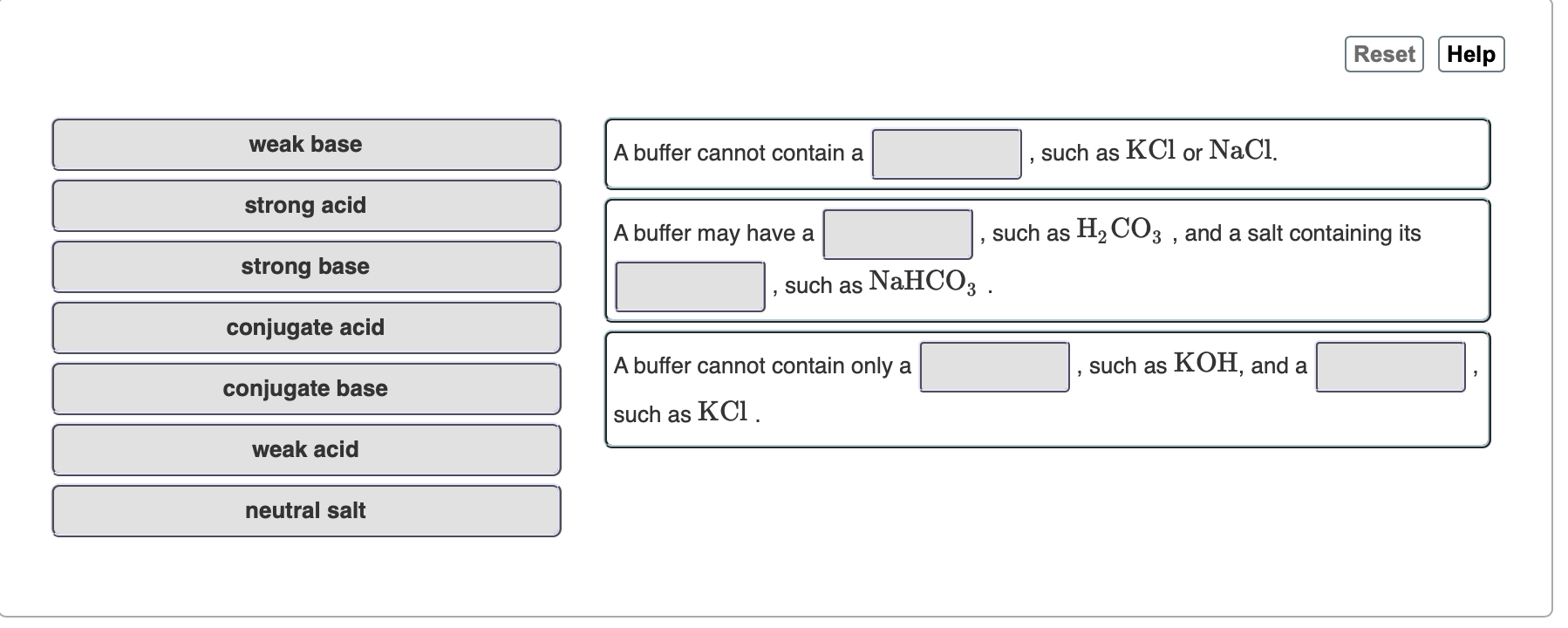
SOLVED: Reset Help weak base buffer cannot contain such as KCl or NaCl strong acid buffer may have such as HzCO: and salt containing its such as NaHCO: strong base conjugate acid

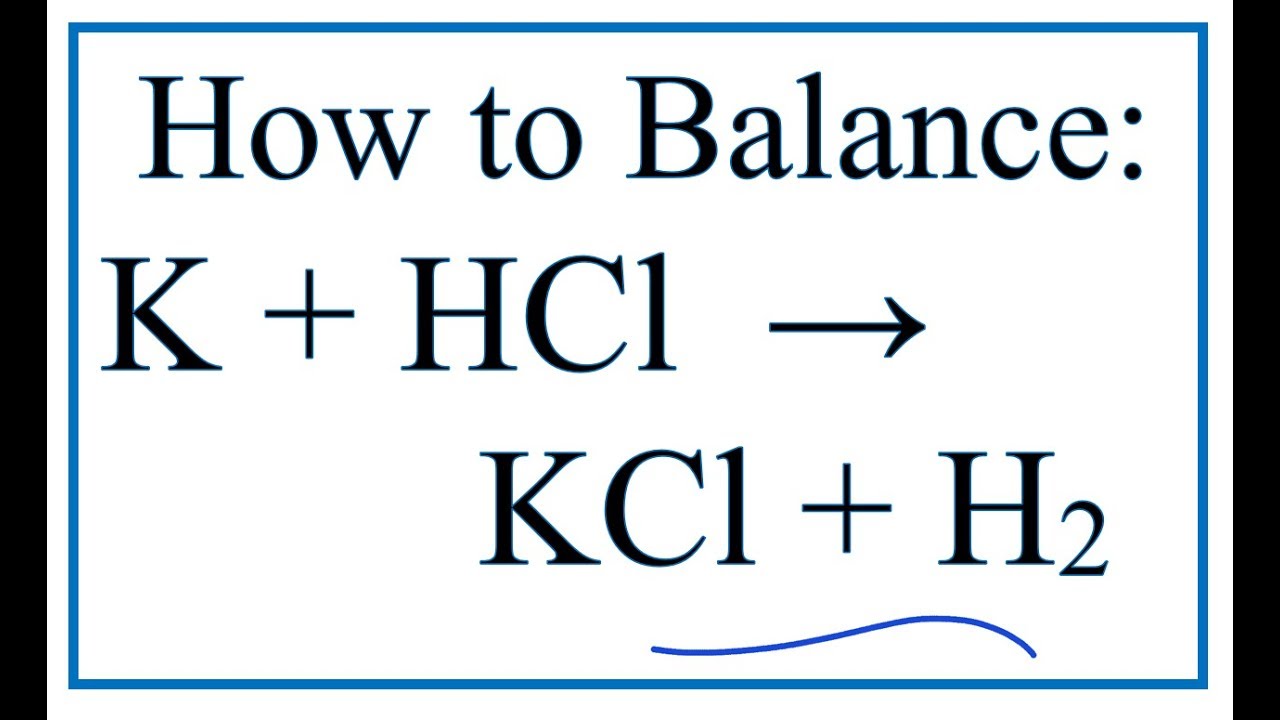
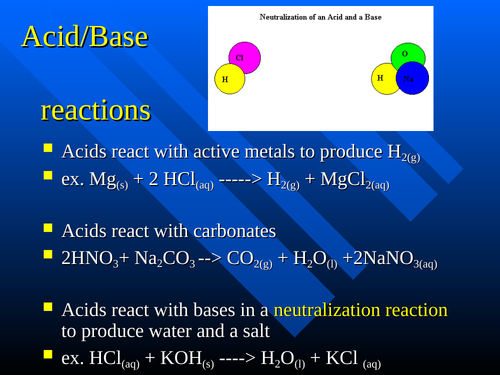
O8-#51-52 BASE ACID SALT WATER 51) KOH + HCl KCl + H 2 O COMBINE THE METAL K FROM THE BASE WITH THE NON METAL Cl FROM THE ACID TO