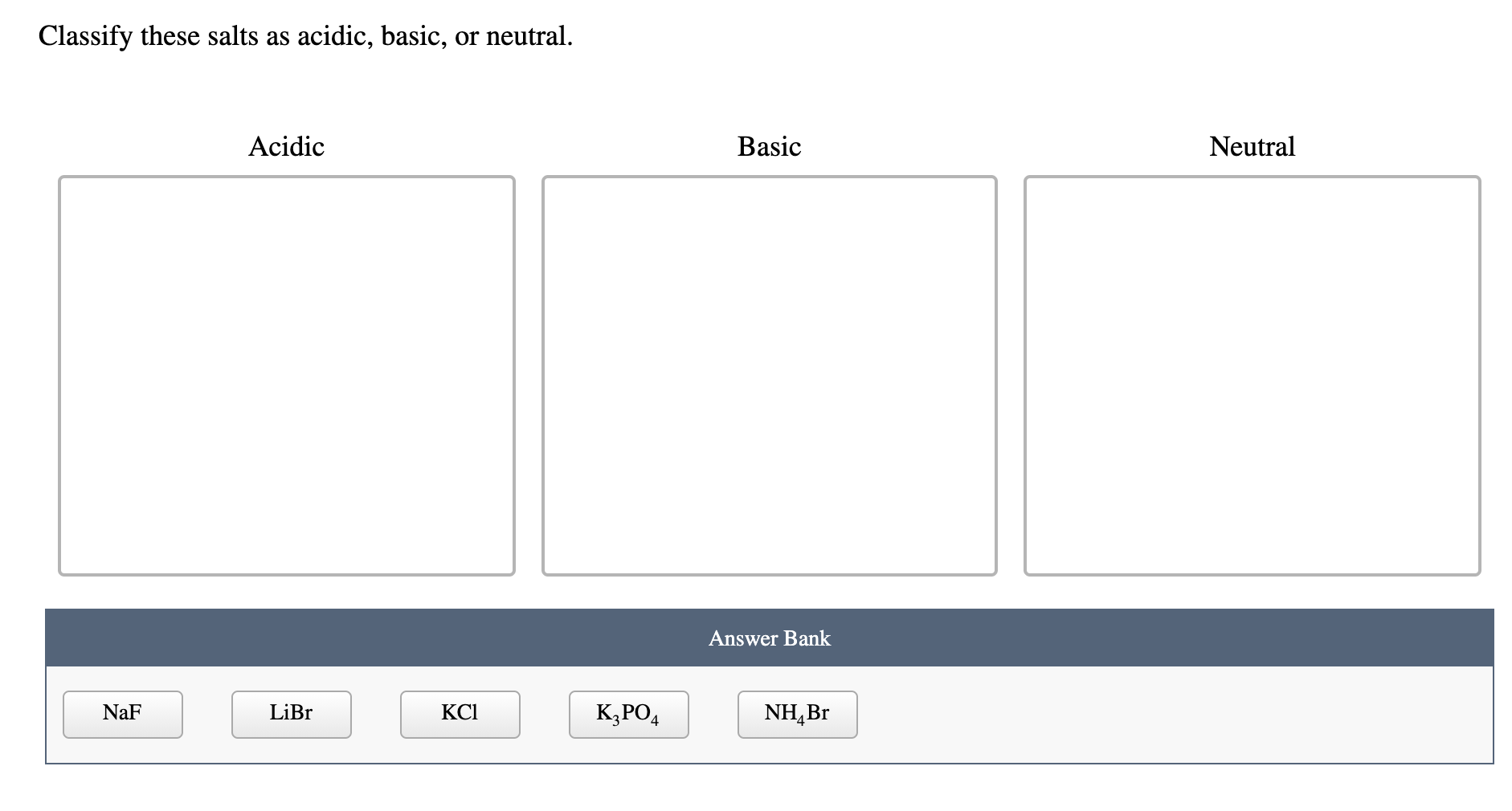
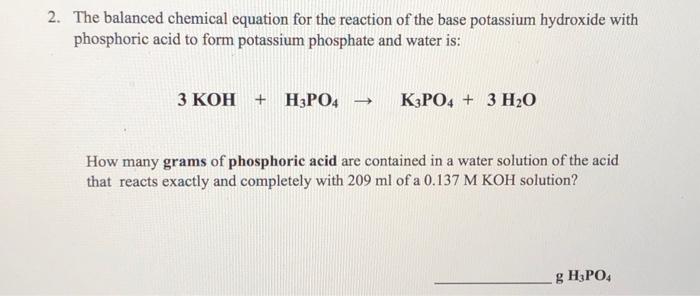
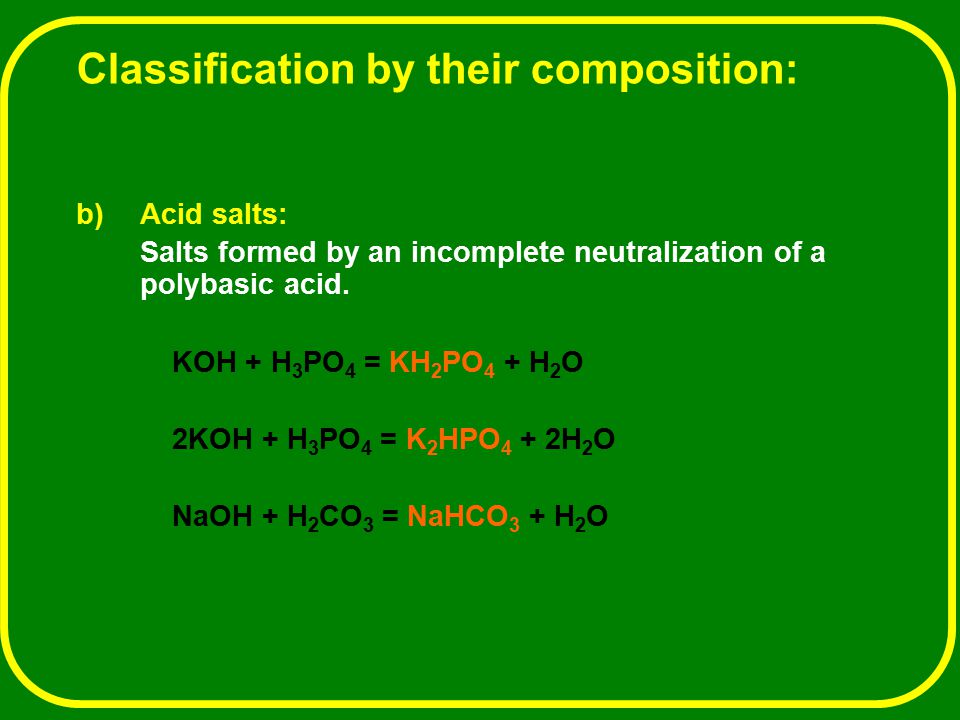
SOLVED: The balanced chemical equation for the reaction of the base potassium hydroxide with phosphoric acid to form potassium phosphate and water is: 3 KOH HPO4 KaPOs 3 HzO How many grams

A NEW METHOD FOR ONE-POT SYNTHESIS OF ARYLOXYPHENOXYPROPIONATE HERBICIDES USING 2,4,6-TRICHLORO-1,3,5-TRIAZINE AND (n-BU)4NI AS A HOMOGENEOUS CATALYST
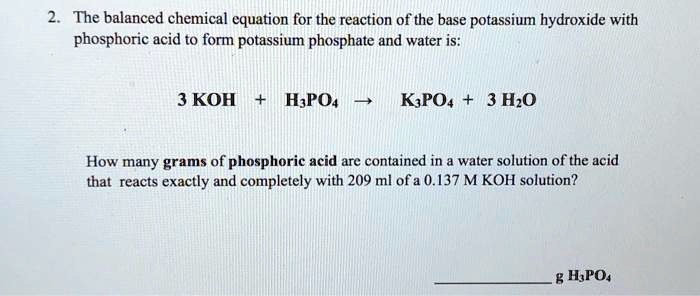
SOLVED: The balanced chemical equation for the reaction of the base potassium hydroxide with phosphoric acid to form potassium phosphate and water is: 3 KOH HPO4 KaPOs 3 HzO How many grams

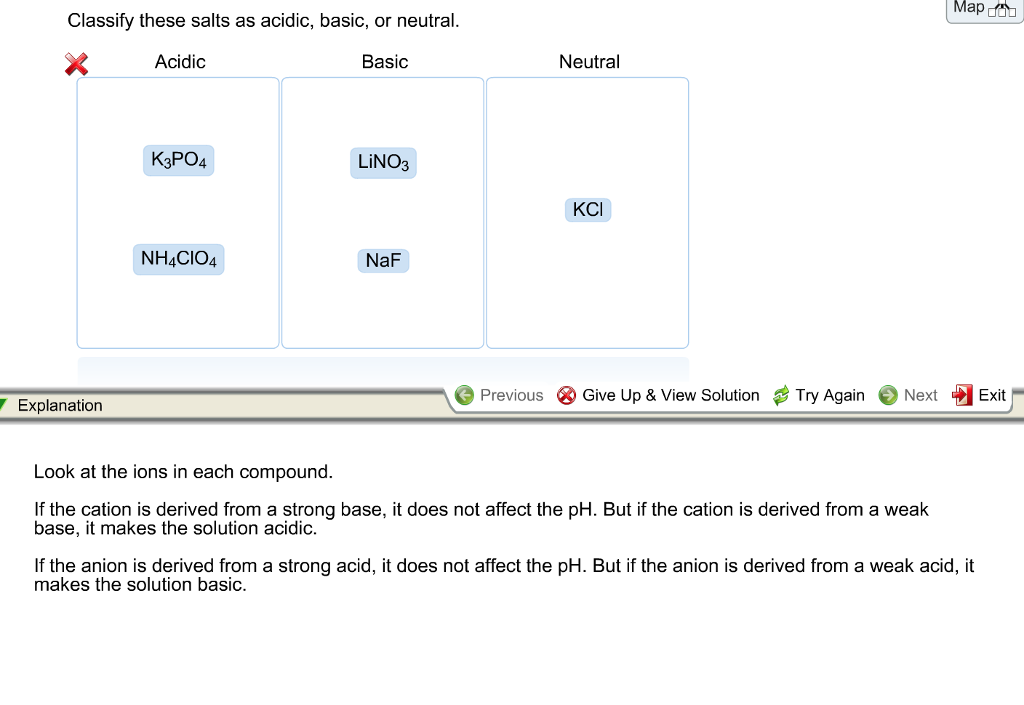
Chapter 19: Acids And Bases. Class question Where can acids be found? –Sodas –Stomach –Vinegar –Citrus fruits Where can bases be found? –Soap –Drano –Antacid. - ppt download

Air-Tolerant Direct Thiol Esterification with Carboxylic Acids Using Hydrosilane via Simple Inorganic Base Catalysis | The Journal of Organic Chemistry

Synthesis of HTLcs modified by K3PO4 for side chain alkylation of toluene with methanol - ScienceDirect

Potassium Phosphate as a High-Performance Solid Base in Phase-Transfer-Catalyzed Alkylation Reactions | Industrial & Engineering Chemistry Research

The potential of K3PO4, K2CO3, Na3PO4 and Na2CO3 as reusable alkaline catalysts for practical application in biodiesel production - ScienceDirect