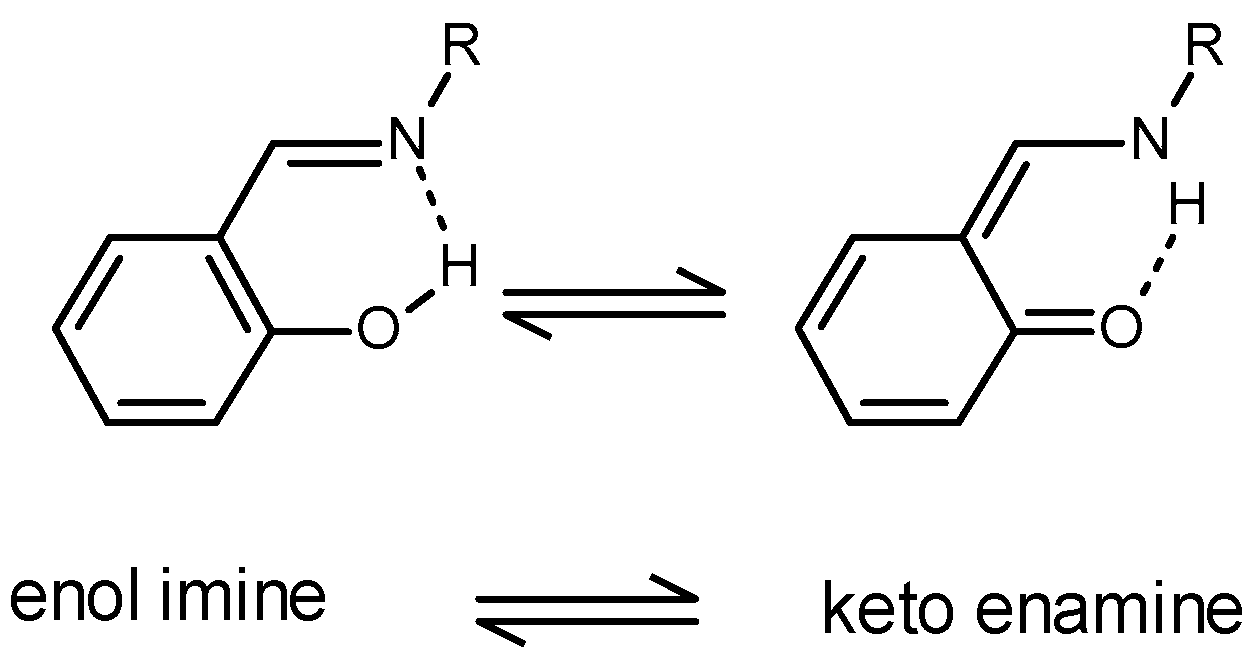
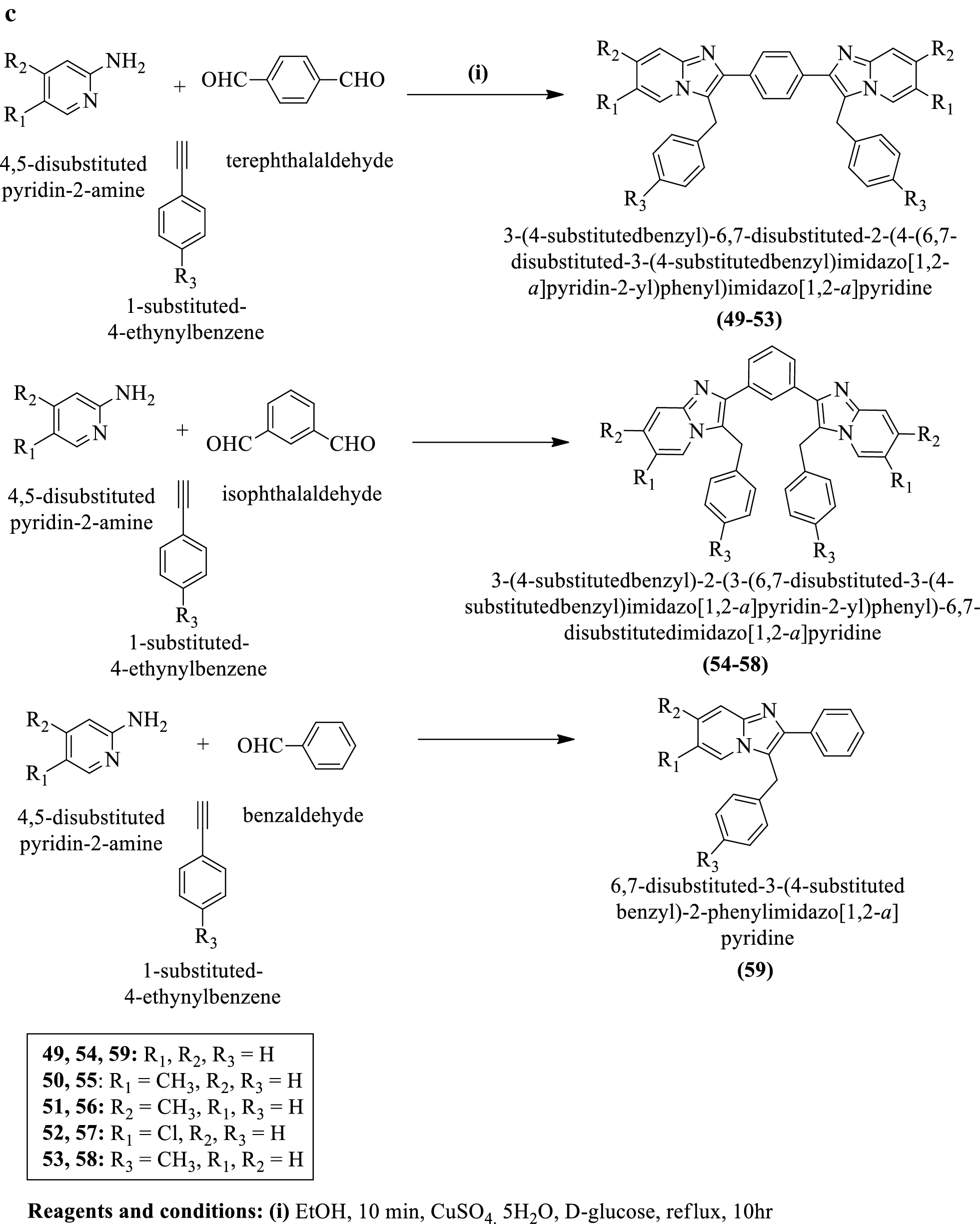
Backbone Boron-Functionalized Imidazoles/Imidazolium Salts: Synthesis, Structure, Metalation Studies, and Fluoride Sensing Properties | Inorganic Chemistry

Crucial Roles of a Pendant Imidazole Ligand of a Cobalt Porphyrin Complex in the Stoichiometric and Catalytic Reduction of Dioxygen - Yang - 2022 - Angewandte Chemie International Edition - Wiley Online Library

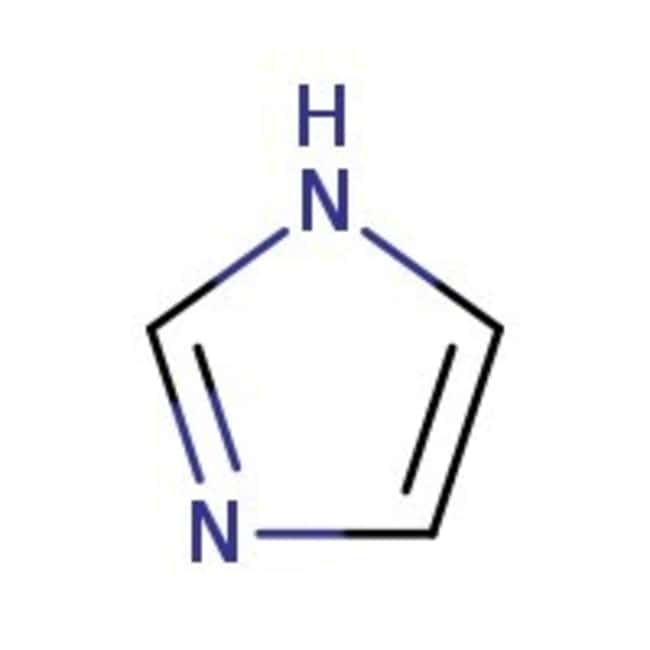
organic chemistry - Does imidazole and hydrochloric acid yield imidazole hydrochloride salt? - Chemistry Stack Exchange

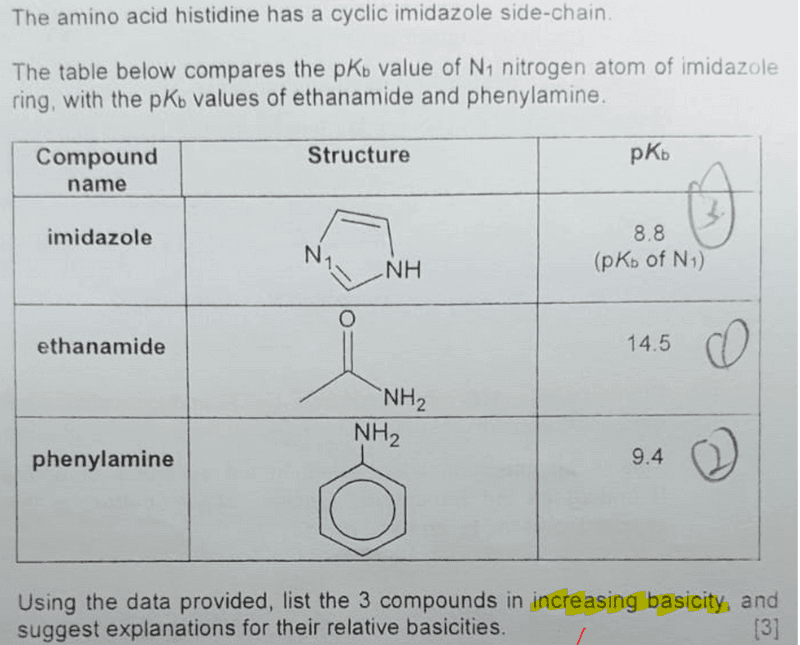
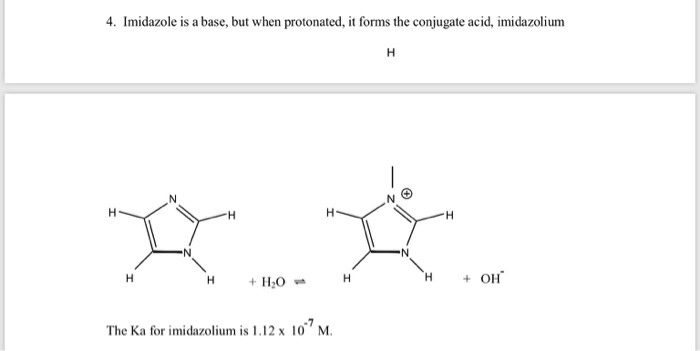
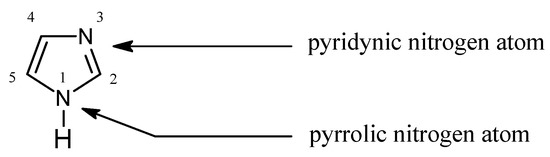
Imidazole forms part of the structure of the amino acid histidine and can act as both an acid and a base. Draw structures for the resonance forms of the products that result

Imidazole forms part of the structure of the amino acid histidine and can act as both an acid and a base. Draw structures for the resonance forms of the products that result
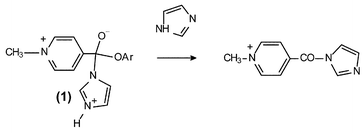
Reaction of imidazole with toluene-4-sulfonate salts of substituted phenyl N-methylpyridinium-4-carboxylate esters: special base catalysis by imidazole - Organic & Biomolecular Chemistry (RSC Publishing)