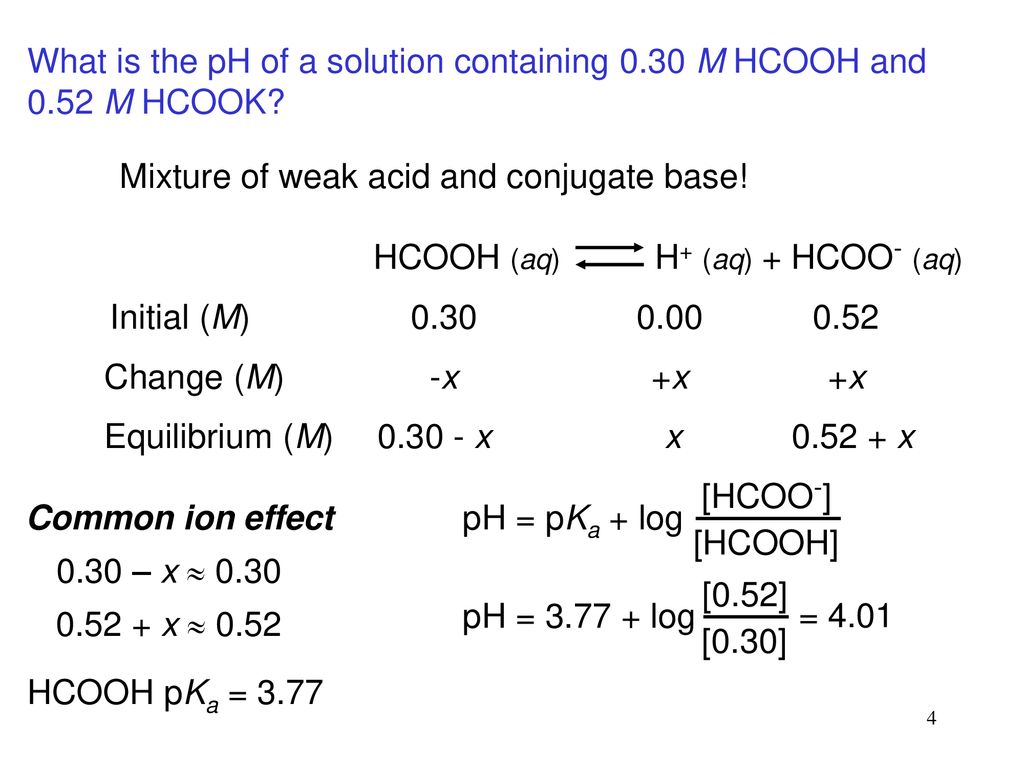
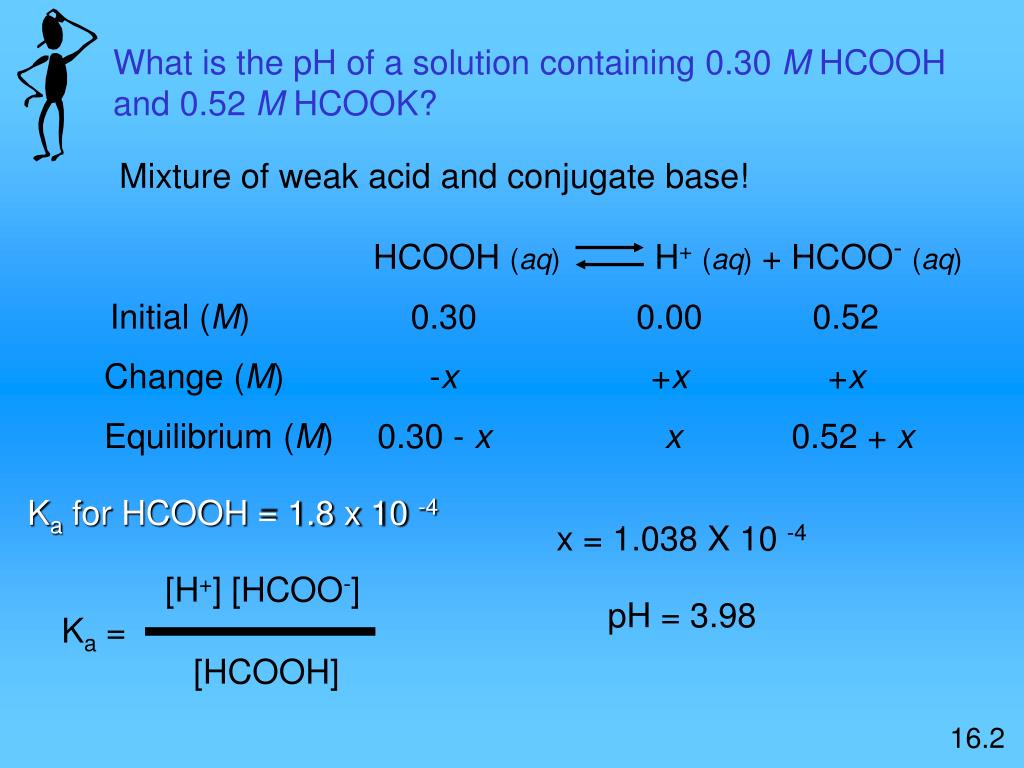
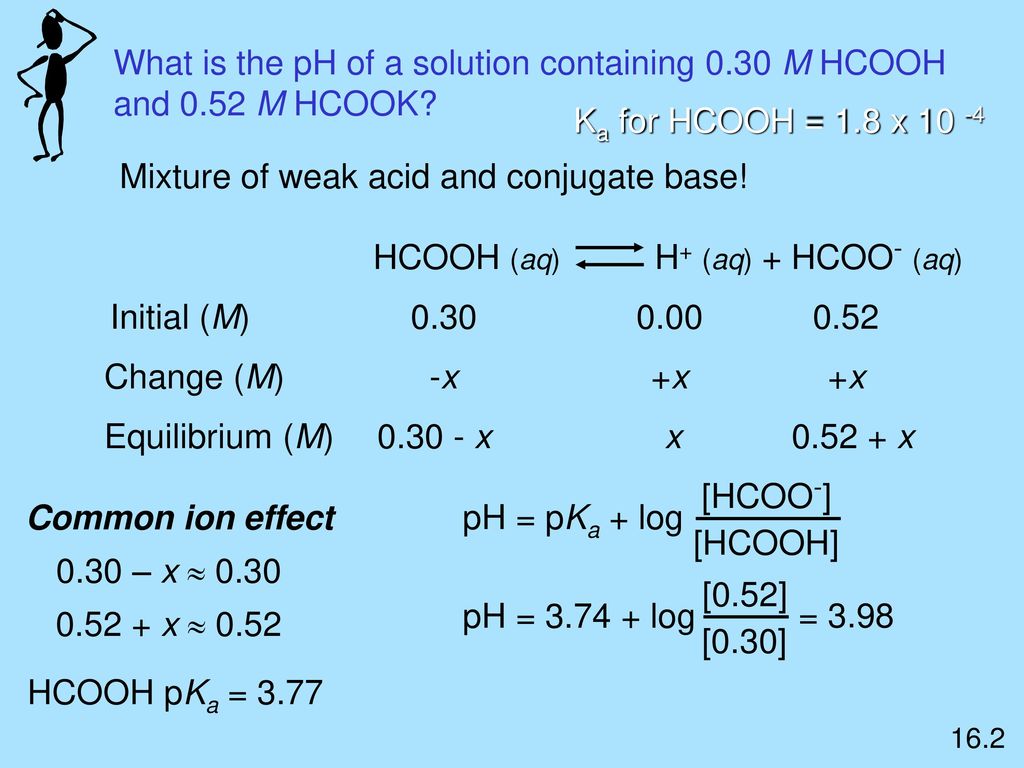
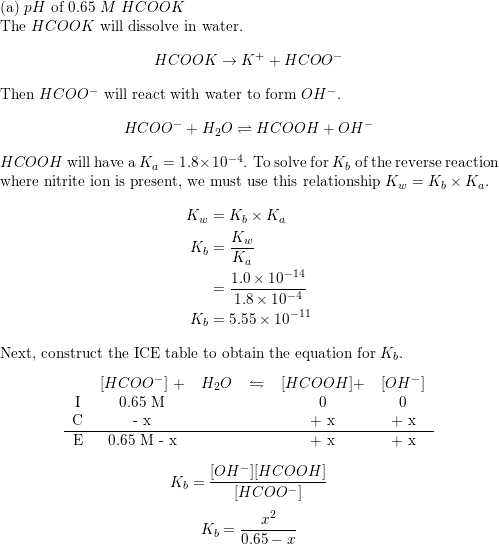A 0.05M HCOOH and 0.1M HCOOK buffer solution was diluted 100 times. How did pH change and why? - Quora
![pH = - log [H + ] or pH = - log [H 3 O + ] Example: If [H + ] = 1 X pH = - log 1 X pH = - (- 10) pH = 10 What would be the pH of a. - ppt download pH = - log [H + ] or pH = - log [H 3 O + ] Example: If [H + ] = 1 X pH = - log 1 X pH = - (- 10) pH = 10 What would be the pH of a. - ppt download](https://images.slideplayer.com/26/8472866/slides/slide_40.jpg)
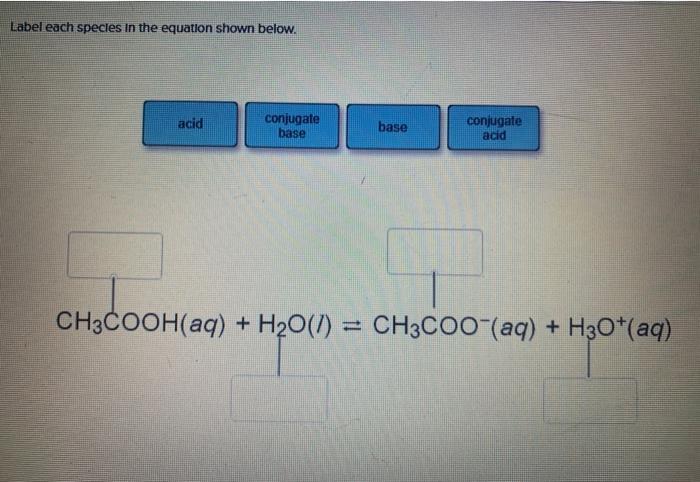
pH = - log [H + ] or pH = - log [H 3 O + ] Example: If [H + ] = 1 X pH = - log 1 X pH = - (- 10) pH = 10 What would be the pH of a. - ppt download
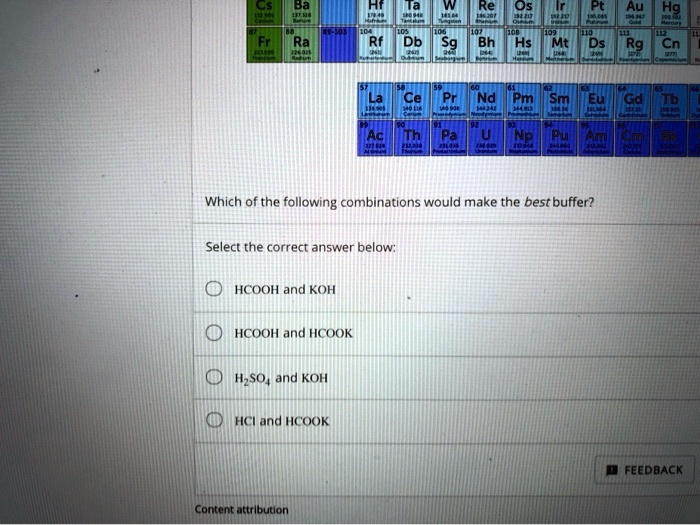
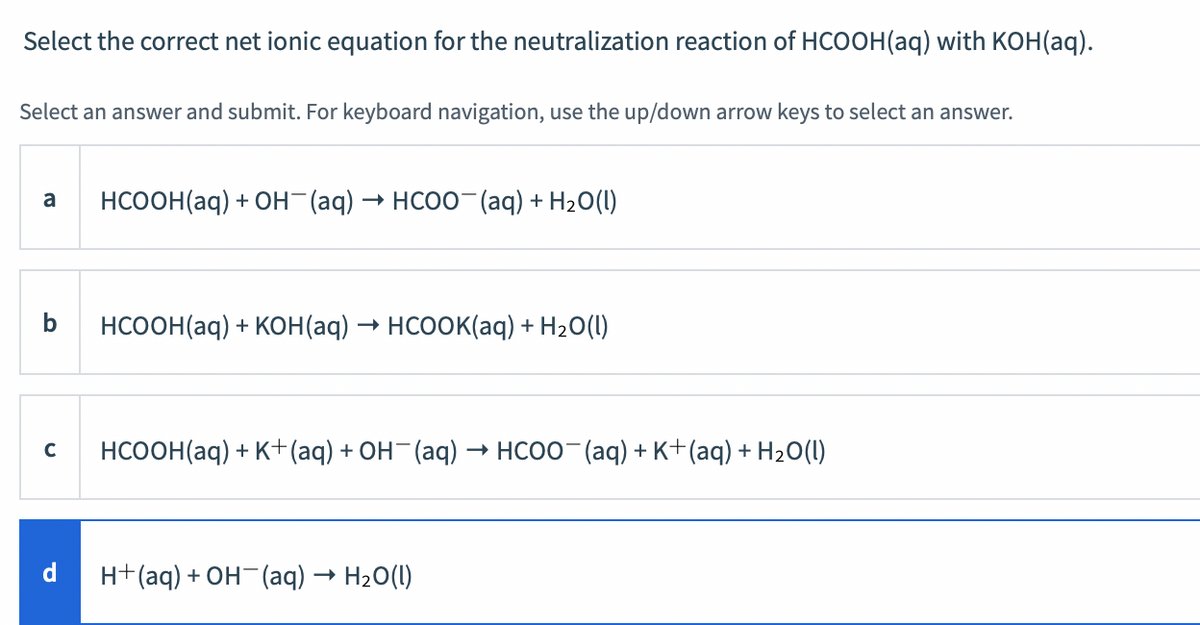
SOLVED: Os 6H Db Sg Hs Mt Ds Rg Cn Cel PN Which of the folllowing combinations would make the best buffer? Select the correct answer below HCOOH and KOH HCOOH and

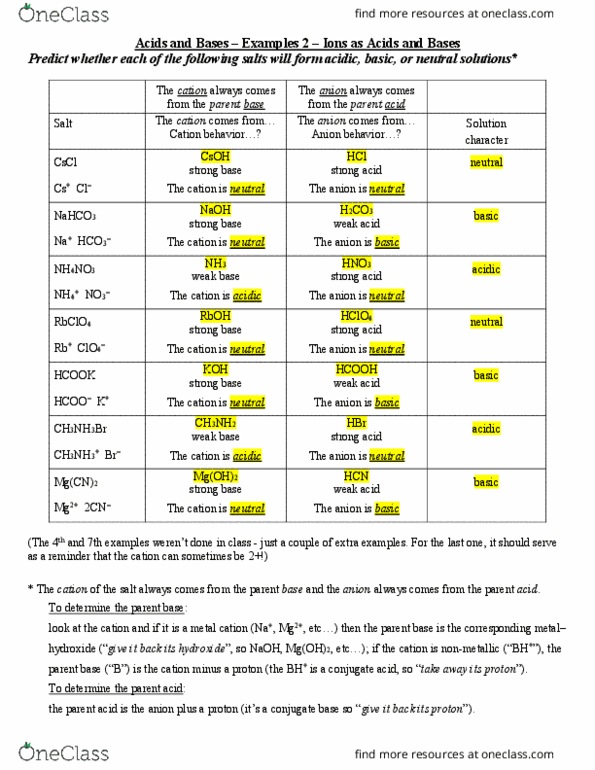
Classify the following salt solution in acid, base and neutral. NaCl, KNO3 , FeCl3 , CuSO4 , CH3COONa, HCOOK, CH3COONH4 , CrCl3 , K2SO4 , Na3PO4 , NH4Cl
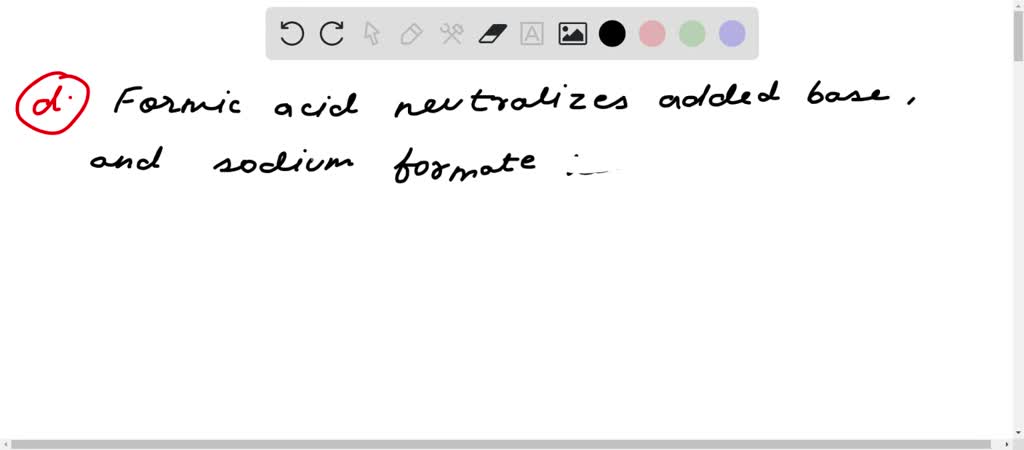
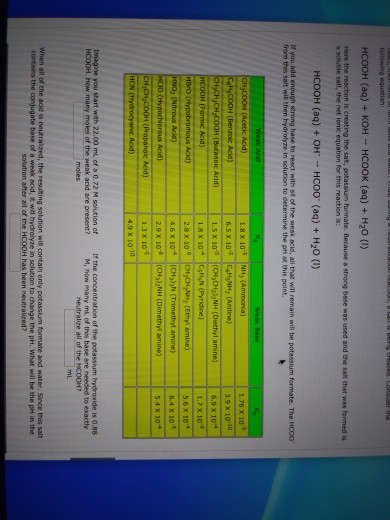
SOLVED: A buffer contains HCOOH (aq) and HCOOK (aq). Which statement correctly summarizes the action of this buffer? Both HCOOH (aq) and HCOOK (aq) neutralize added acid. Both HCOOH (aq) and HCOOK (

Why an aqueous solution of NH4Cl is acidic while that of HCOOK is basic - Chemistry - Ionic Equilibria - 16488273 | Meritnation.com

EHSQ (Environment,Health,Safety and Quality) : Question : Is HCOOK an acid or base or neutral ? Answer : HCOOK ( Potassium formate ) is base

EHSQ (Environment,Health,Safety and Quality) : Question : Is HCOOK an acid or base or neutral ? Answer : HCOOK ( Potassium formate ) is base
Steady continuous dosage of FA. Reaction conditions: HCOOH (5 mmol),... | Download Scientific Diagram

Effect of HCOOK/Ethanol on Fe/HUSY, Ni/HUSY, and Ni–Fe/HUSY Catalysts on Lignin Depolymerization to Benzyl Alcohols and Bioaromatics | ACS Omega