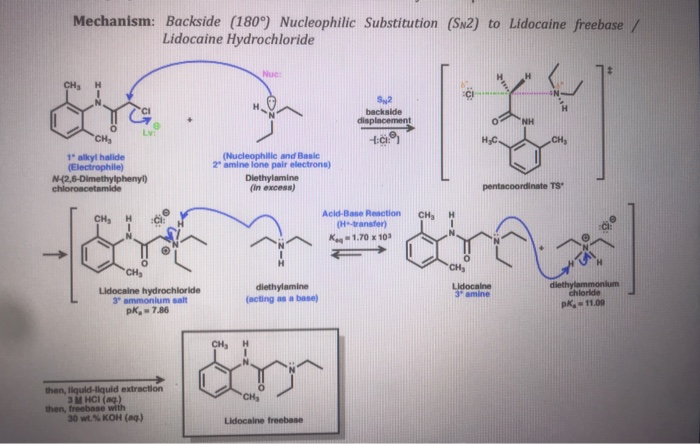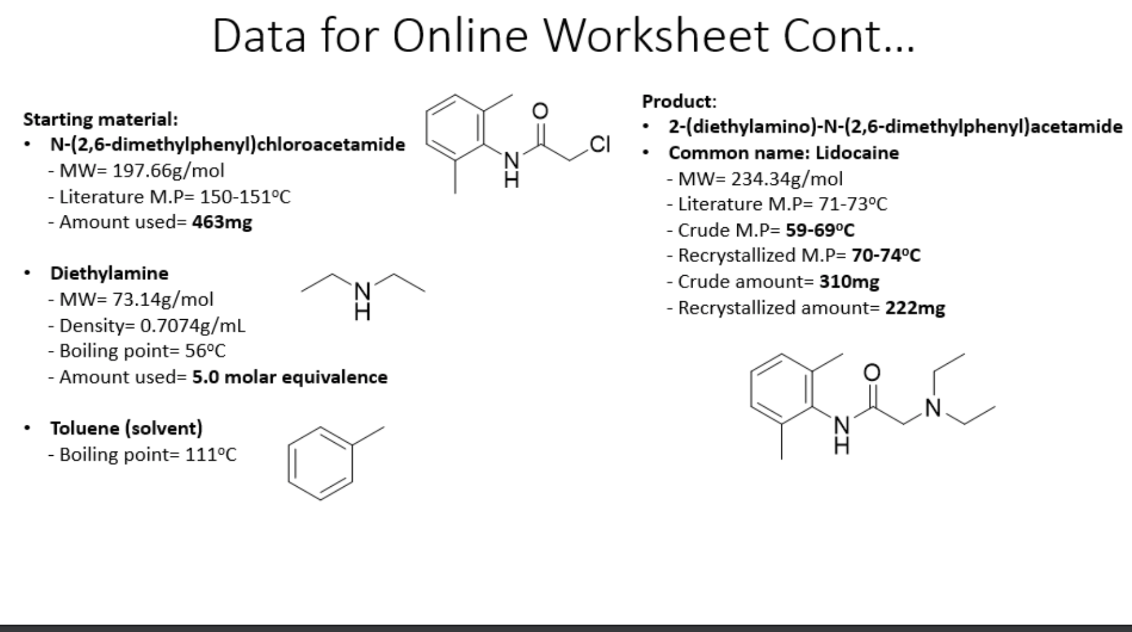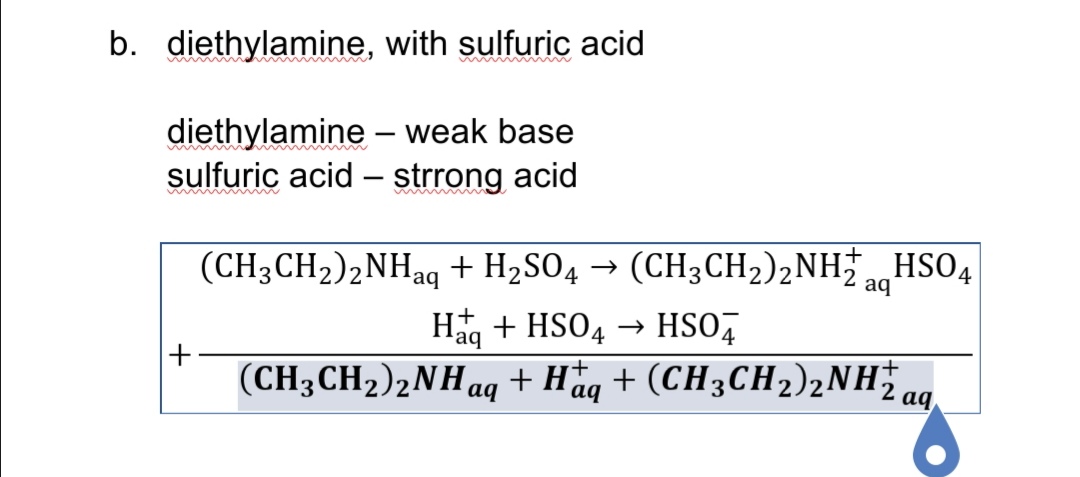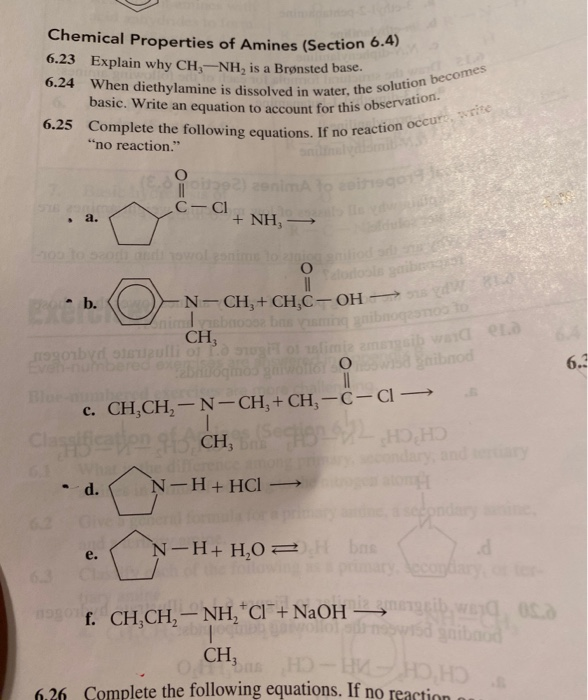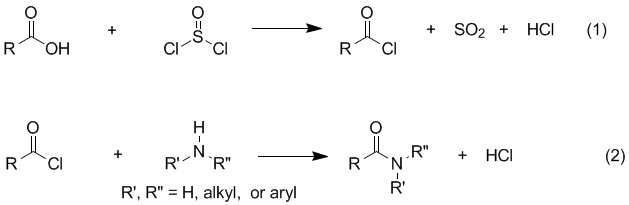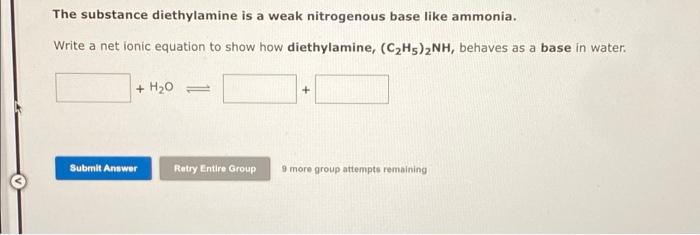
DICLOFENAC DIETHYLAMINE + LINSEED OIL + METHYL SALCILATE + MENTHOL +BENZYL ALCOHOL GEL BASE, ALLEN DALE BIOSCIENCES, Panchkula
The pH of 0.05 M aqueous solution of diethyl amine is 12.0. Calculate Kb. - Sarthaks eConnect | Largest Online Education Community