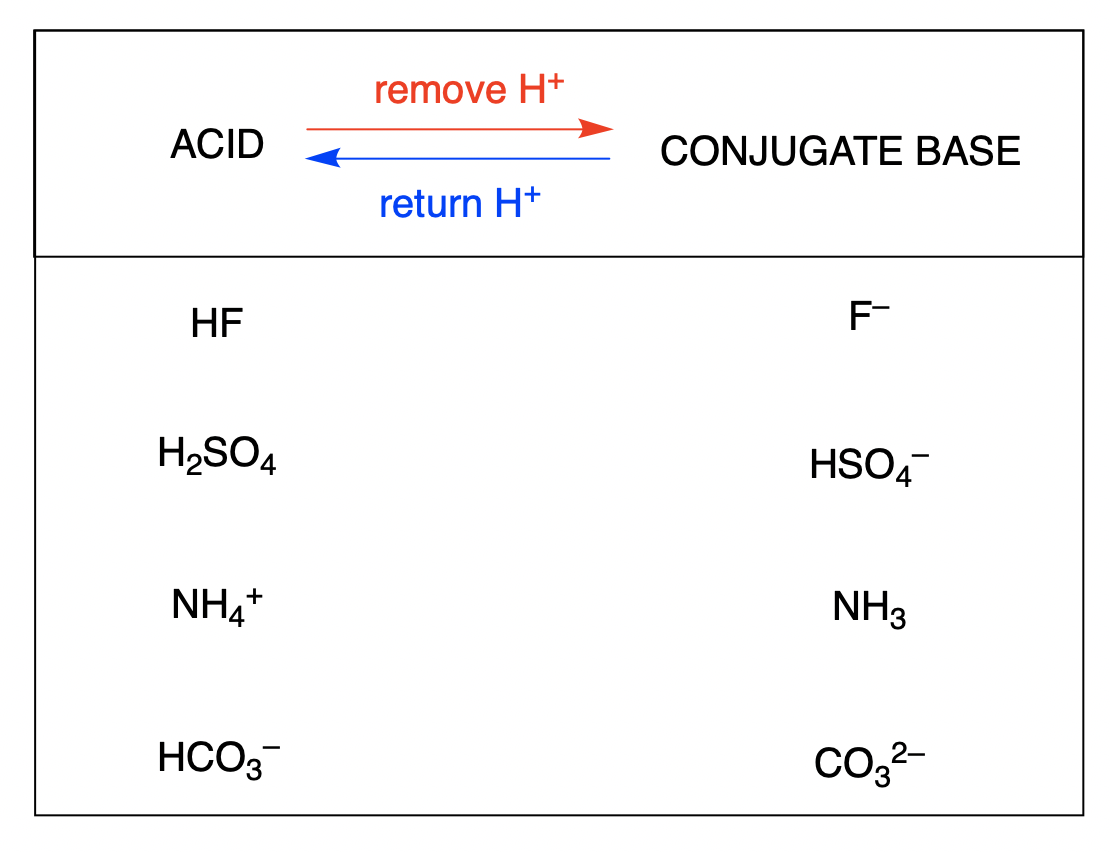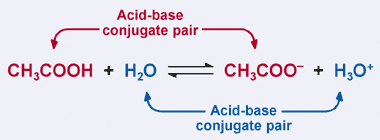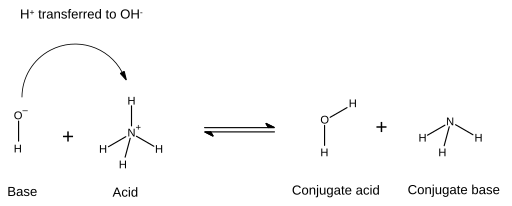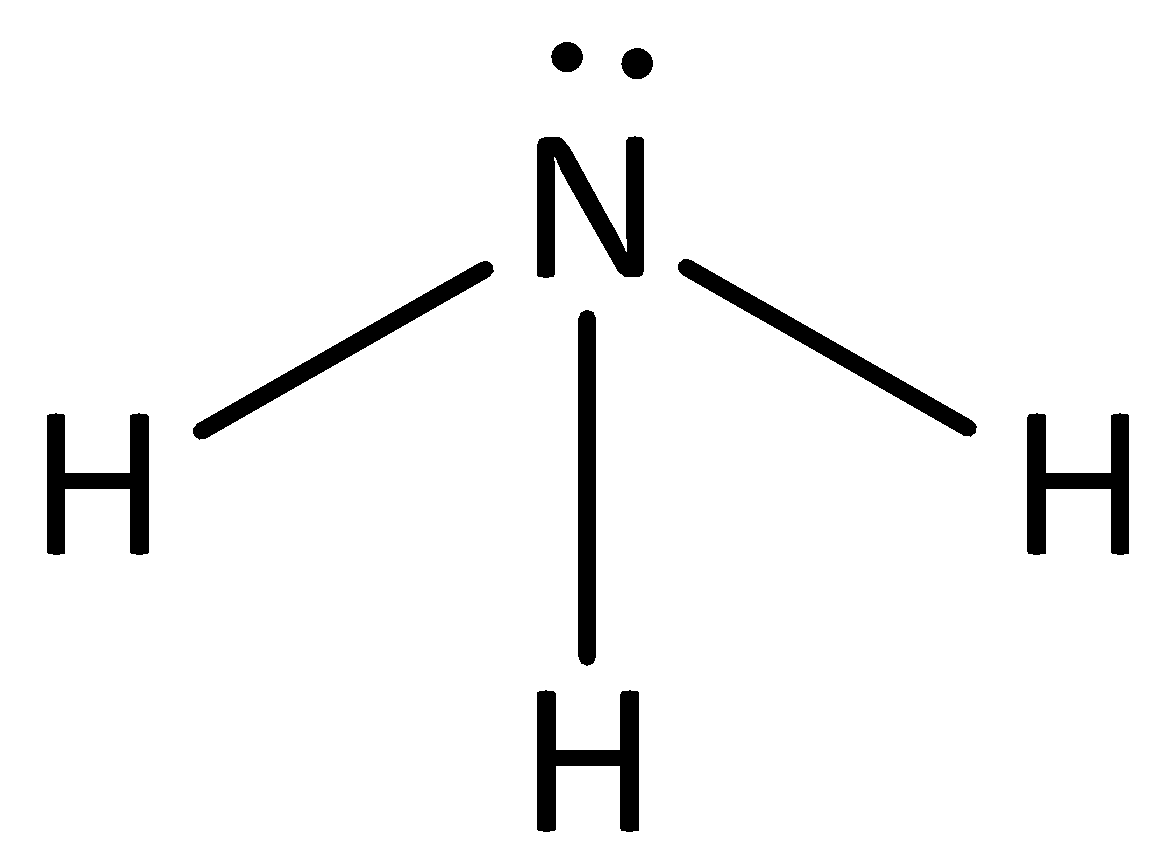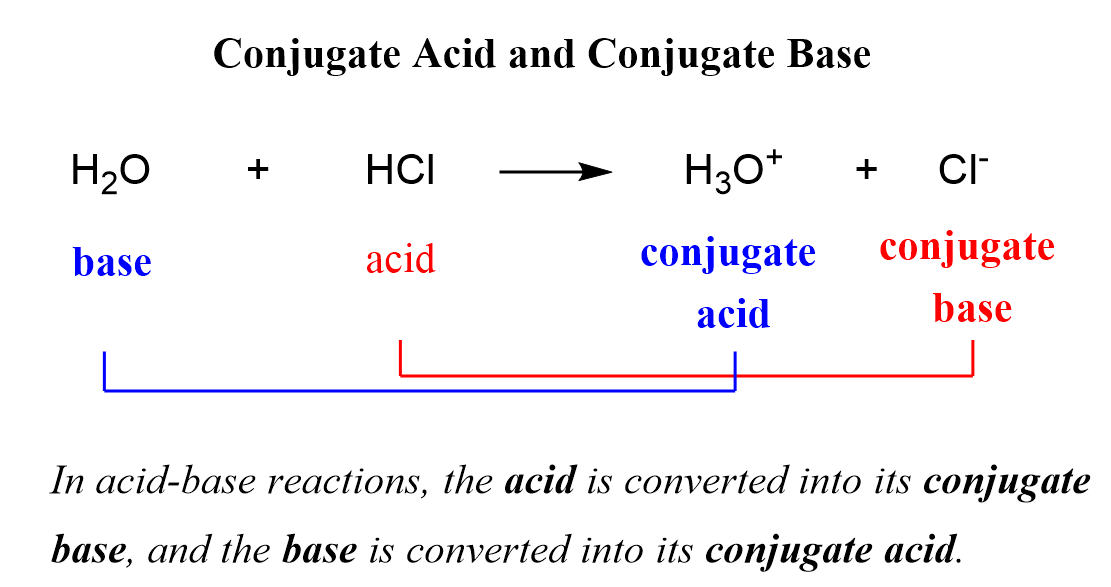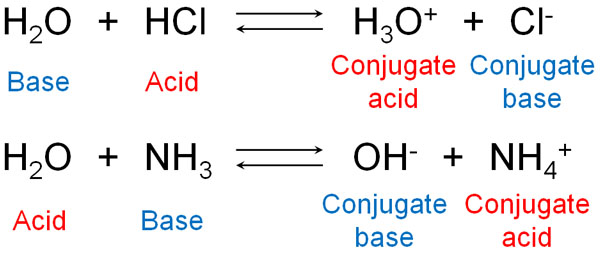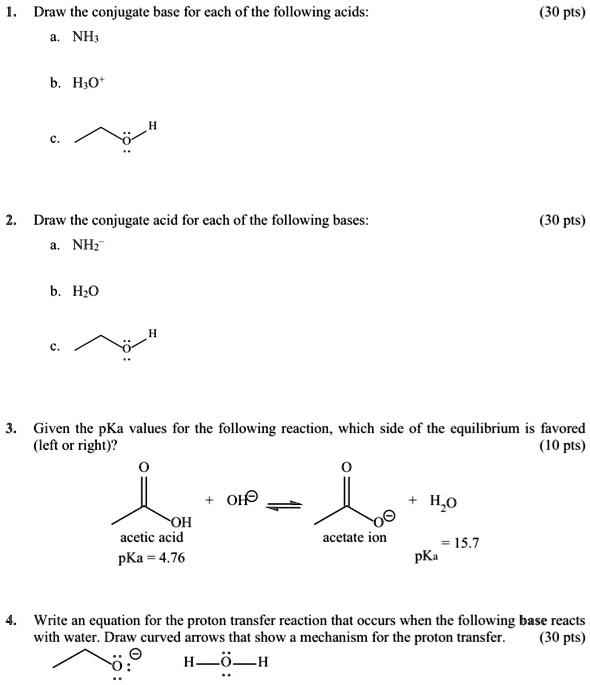
SOLVED: Draw the conjugate base for cach of the following acids: NH; (30 pts) H;O - Draw the conjugate acid for each of the following bases: NH; (30 pts) Hjo Given the

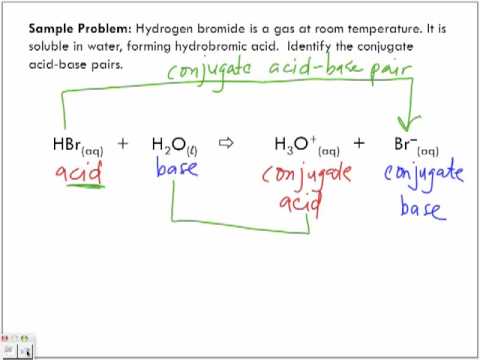
How to Identify Acid, Base, Conjugate Acid, and Conjugate Base Examples and Practice Problems - YouTube

Use your understanding of molecular structure to explain why the conjugate bases of acids like formic acid CHOOH, acetic acid CH3COOH, and phosphoric acid are only stable enough to be weak acids;
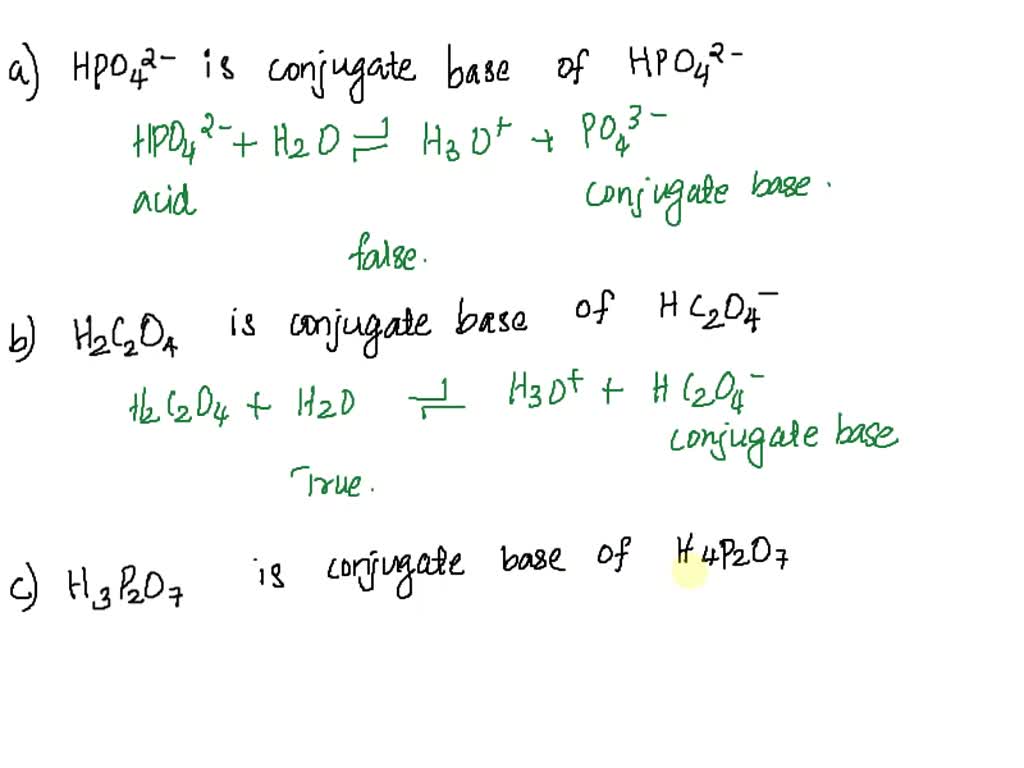
SOLVED: Using your understanding of conjugate acids and bases pick the correct statement from the multiple choices. a) HPO42- is the conjugate base of HPO42-. b) H2C2O4 is the conjugate base of

explanation 1) Look up the chemical name of aspirin and then its conjugate base (as the sodium salt) and provide those names here 2)The manufacturer's label for the "aspirin lists aspirin as