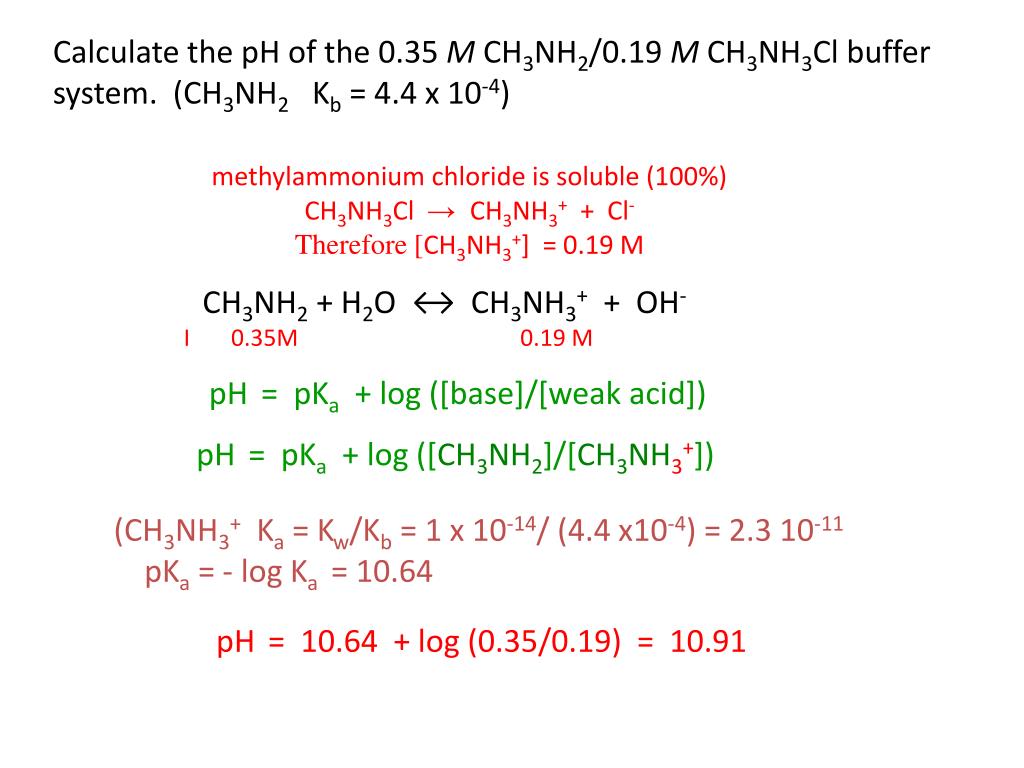
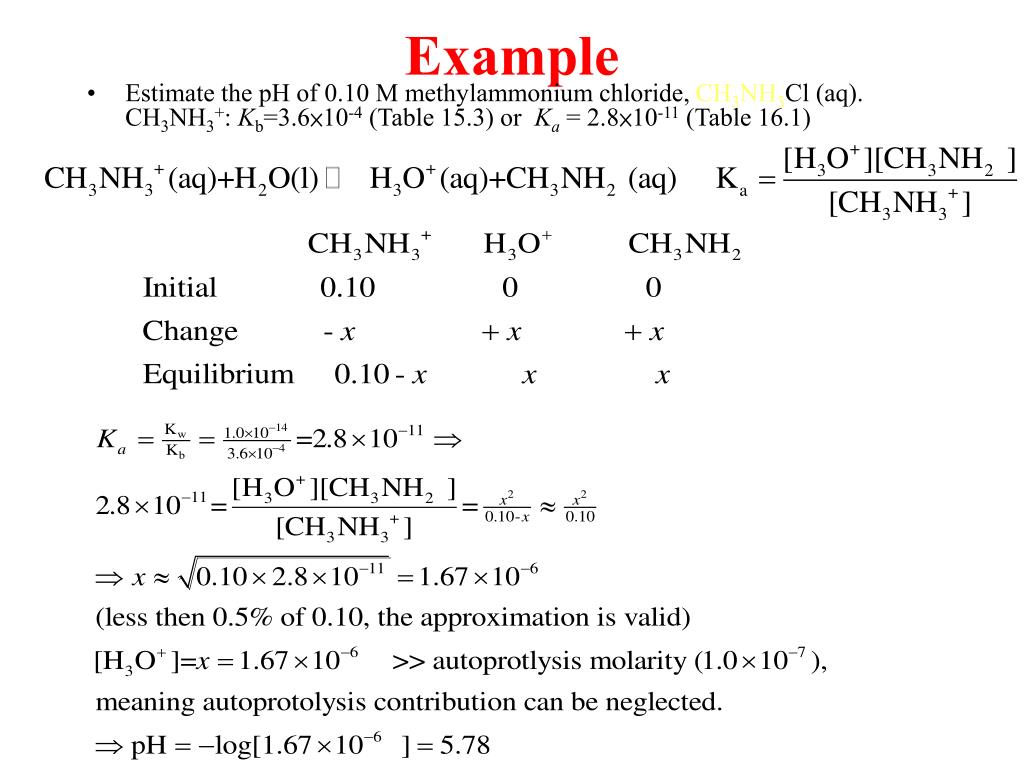
CH3NH3Cl-Assisted One-Step Solution Growth of CH3NH3PbI3: Structure, Charge-Carrier Dynamics, and Photovoltaic Properties of Perovskite Solar Cells | The Journal of Physical Chemistry C

Solved) - Is the solution of KCI acidic, basic or neutral?. Is the solution... (1 Answer) | Transtutors
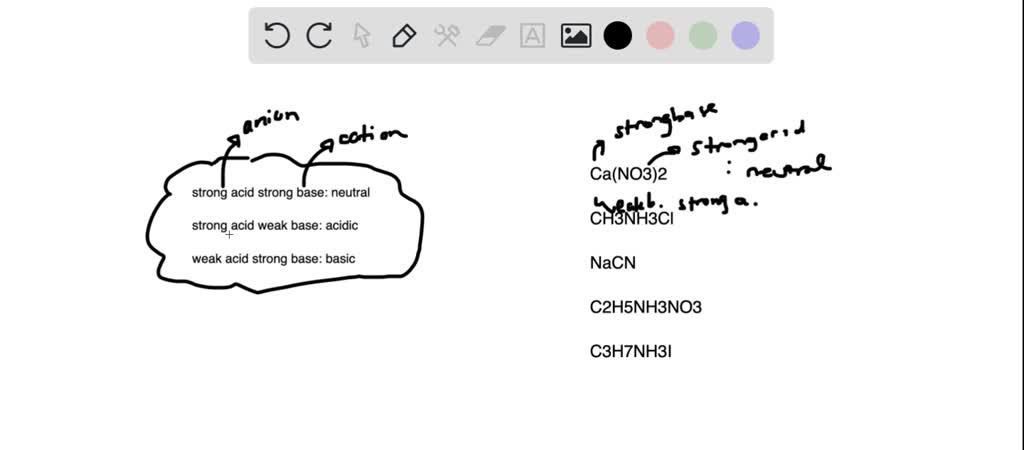
SOLVED: Consider 0.25 M solutions of the following salts. For each salt, indicate whether the solution is acidic, basic, or neutral. Ca(NO3)2 CH3NH3Cl NaCN C2H5NH3NO3 C3H7NH3I
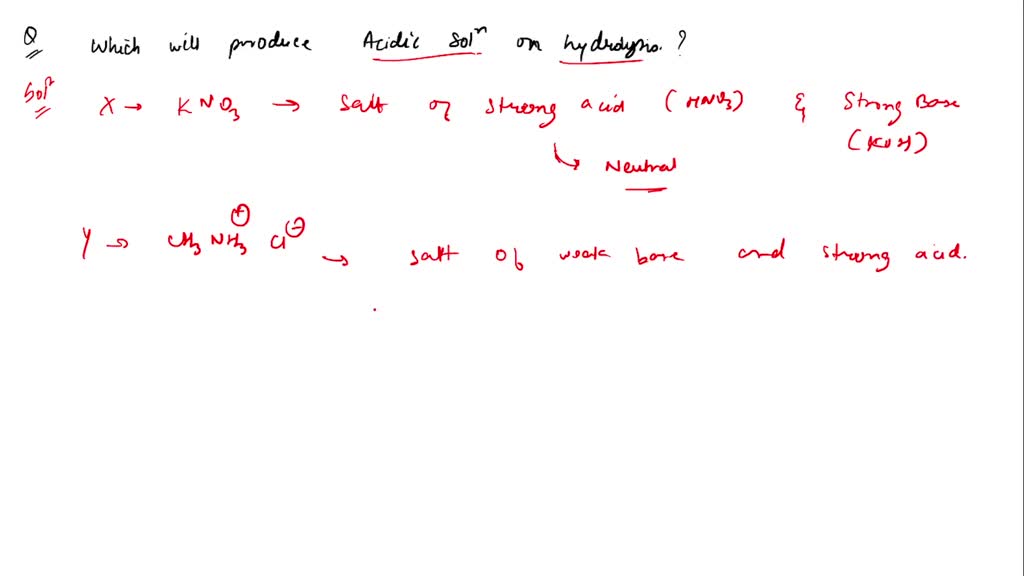
SOLVED: Which of the following compounds, when dissolved in water will produce an acidic solution? X. KNO3 Y. CH3NH3Cl Z. NaHSO4 X only X and Y Y and Z (Correct answer) Z

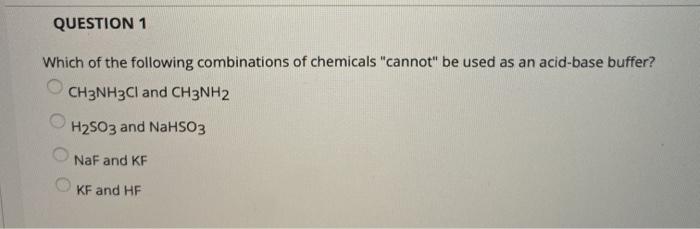
SOLVED: Rank the following salts in order of increasing pH a) NaNO2, CsCl, CH3NH3Cl b) Ca(ClO4), K2S, NH4NO3

Write the net ionic reaction that occurs upon the addition of hno3 to a solution which contains methylamine - Brainly.com

OneClass: please write an EQUILIBRIUM reaction that shows CH3NH3Cl asacidic- Write a chemical equatio...

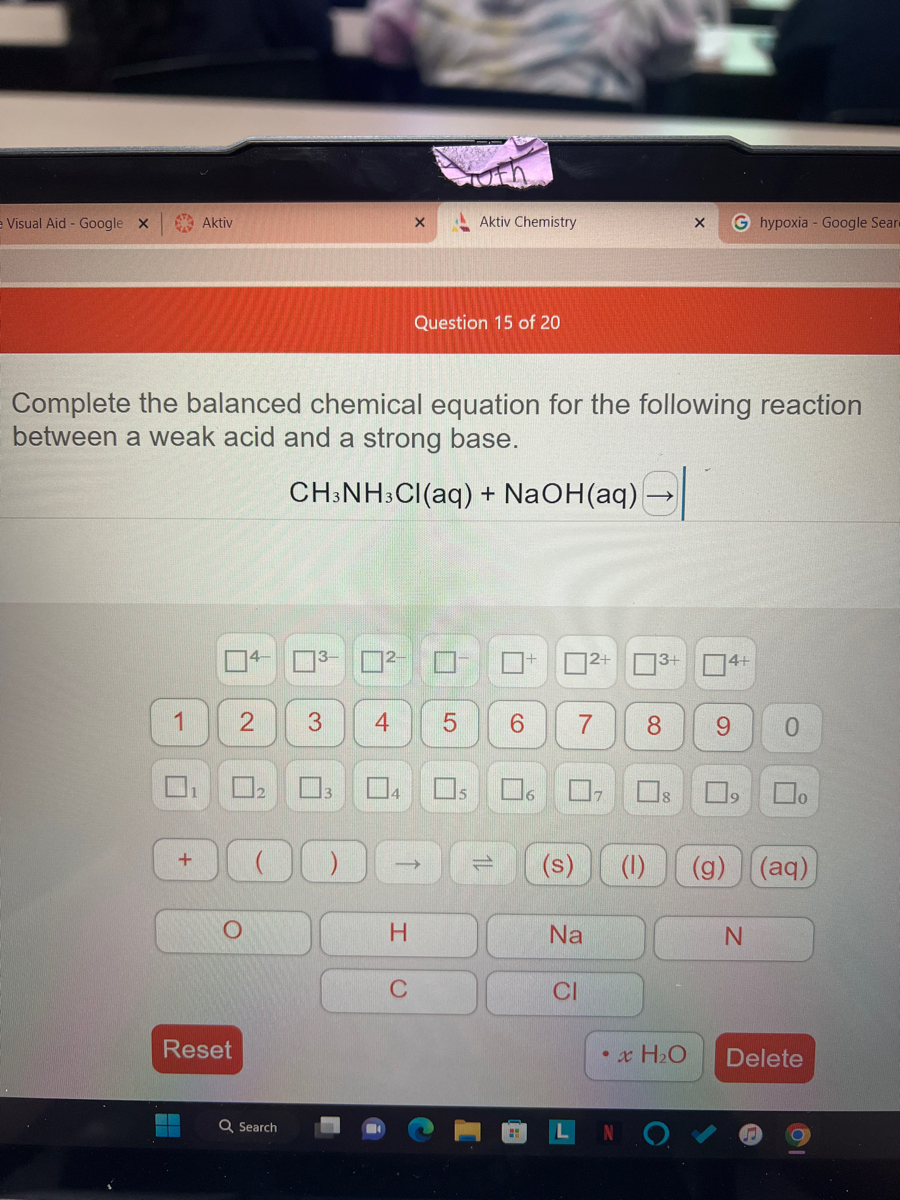
SOLVED: Methylamine, CH3NH2, and methylammonium chloride, CH3NH3Cl write the identity of the two species in each set (strong acid/strong base/weak acid/weak base/salt/other) and explain reason. Identify if anything in common betweenthe two









![14.82 | What is [OH−] in a solution of 0.125 M CH3NH2 and 0.130 M CH3NH3Cl? - YouTube 14.82 | What is [OH−] in a solution of 0.125 M CH3NH2 and 0.130 M CH3NH3Cl? - YouTube](https://i.ytimg.com/vi/rLbt_7_Q8r4/maxresdefault.jpg)