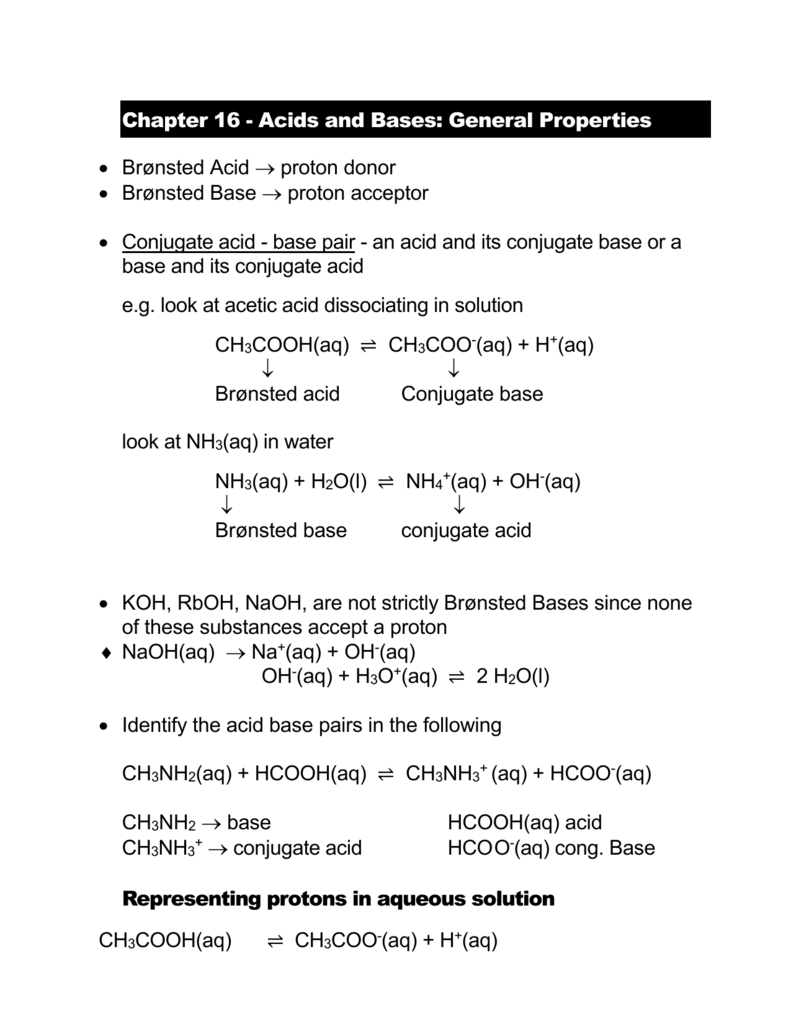
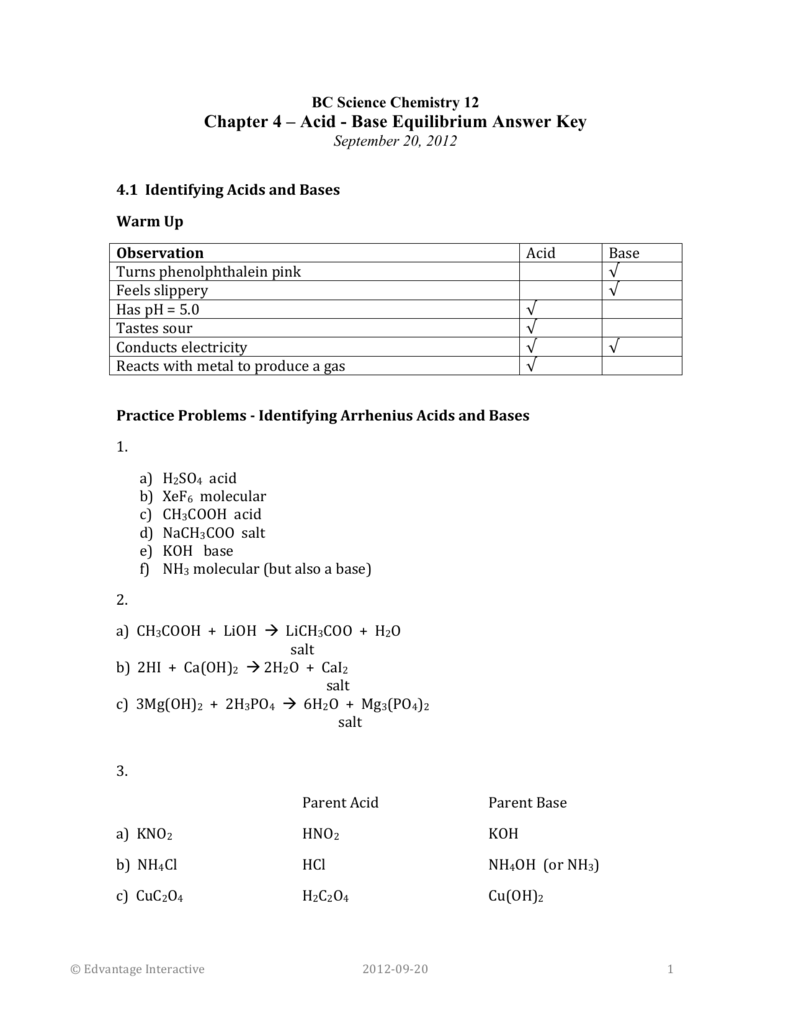
Complete the below acid-base reaction and name the salt formed. (a) Et3N+HCl gives to (b) C5H11NH+CH3COOH gives to | Homework.Study.com

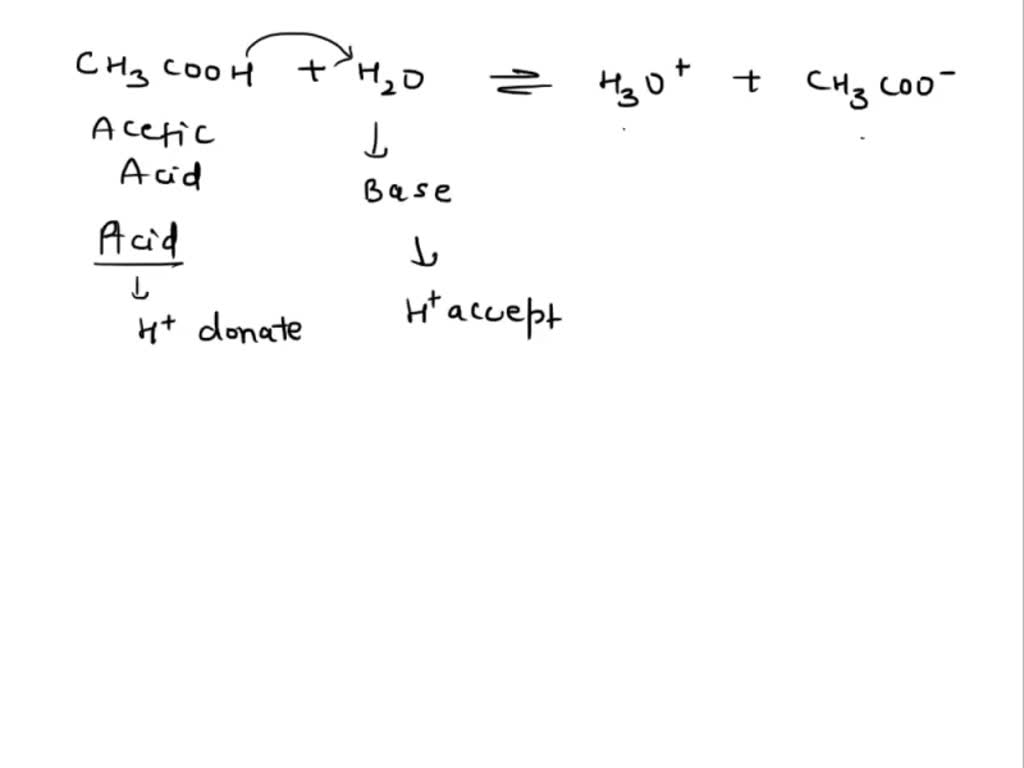
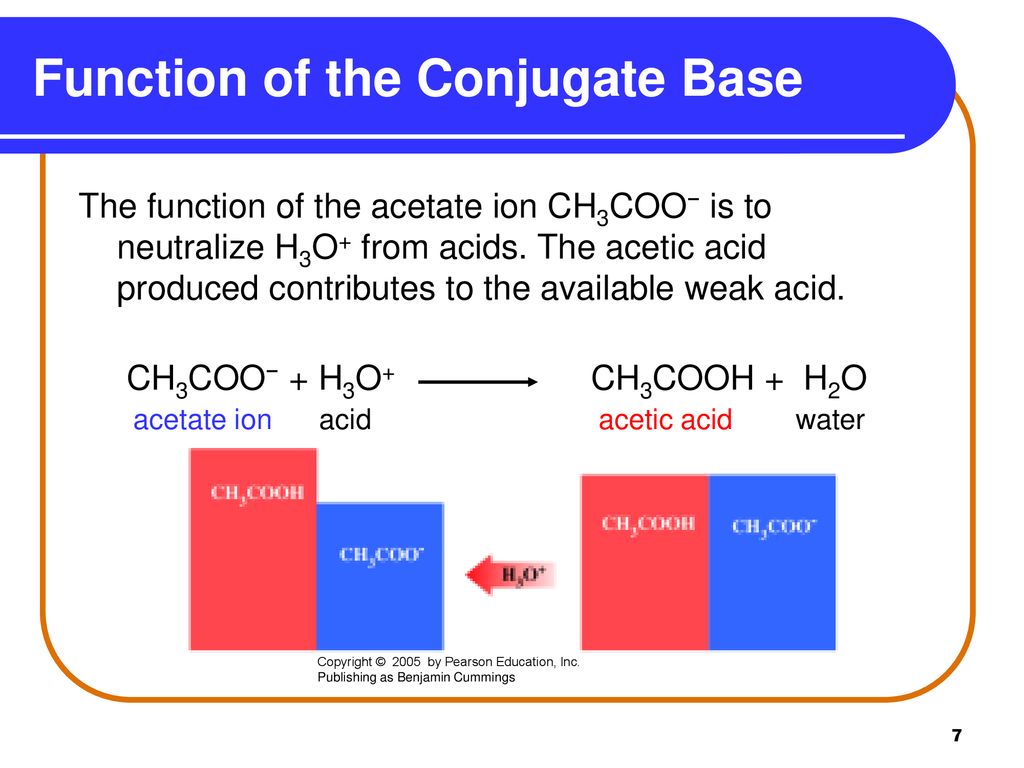
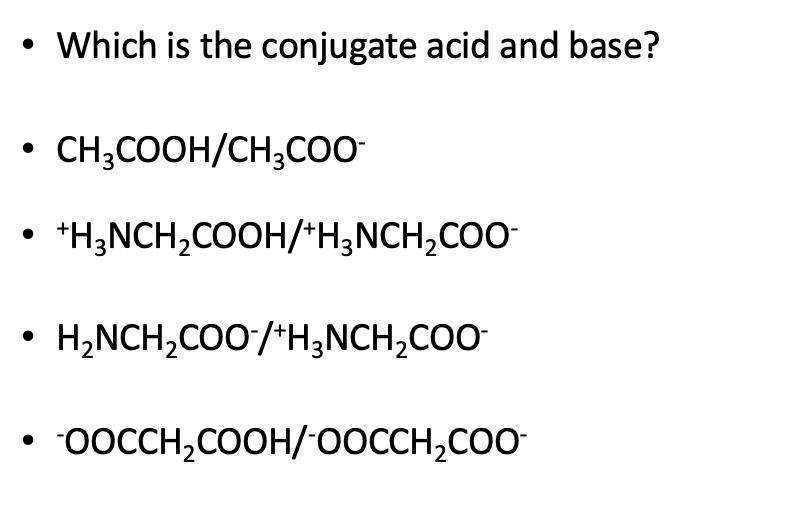
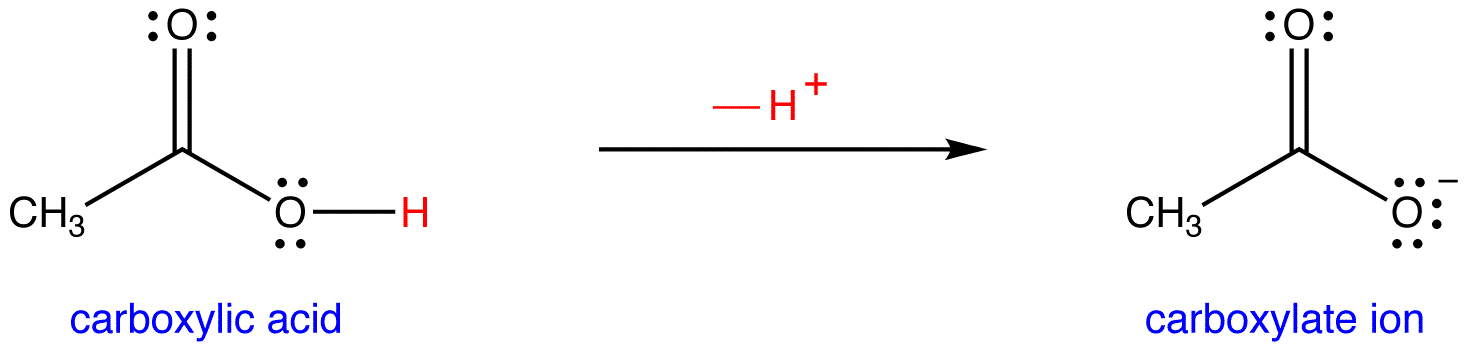
Ionic dissociation of acetic acid is represented as CH3COOH + H2O iff H3O^(+) + CH3COO^- Which of the following statement are/is correct? 1. According to Lowery and Bronsted, the reaction posses an
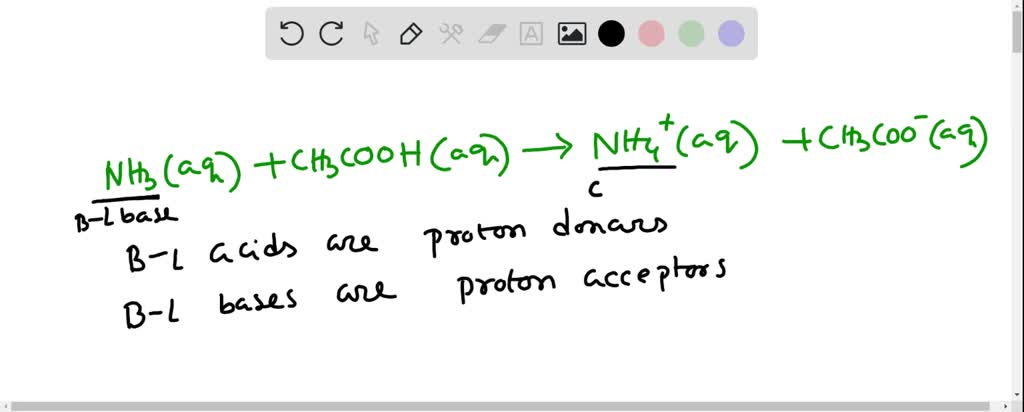
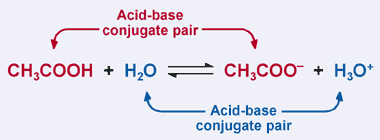
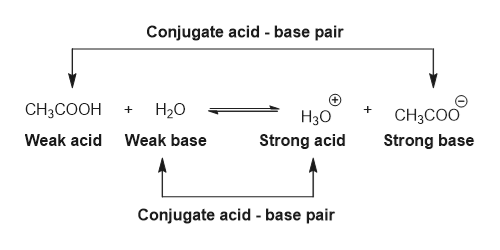
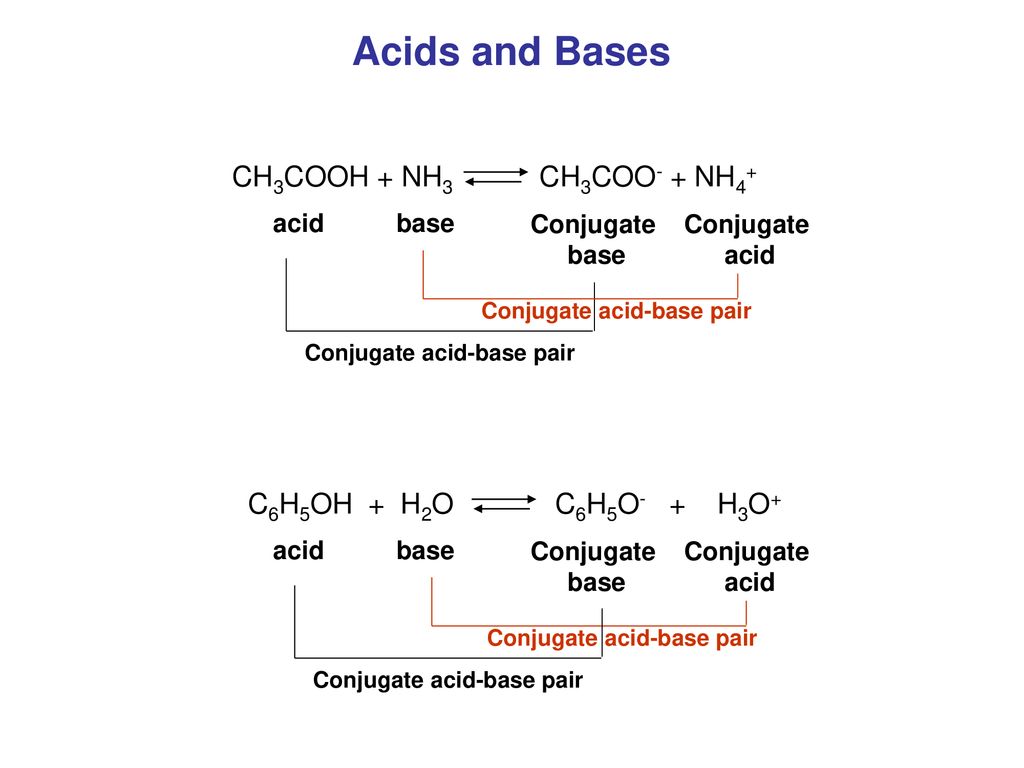
SOLVED: Identify the acid, base, conjugate acid and conjugate base in the following reactions: 1. NH3(aq) + CH3COOH(aq) —> NH4+(aq) + CH3COO- (aq)
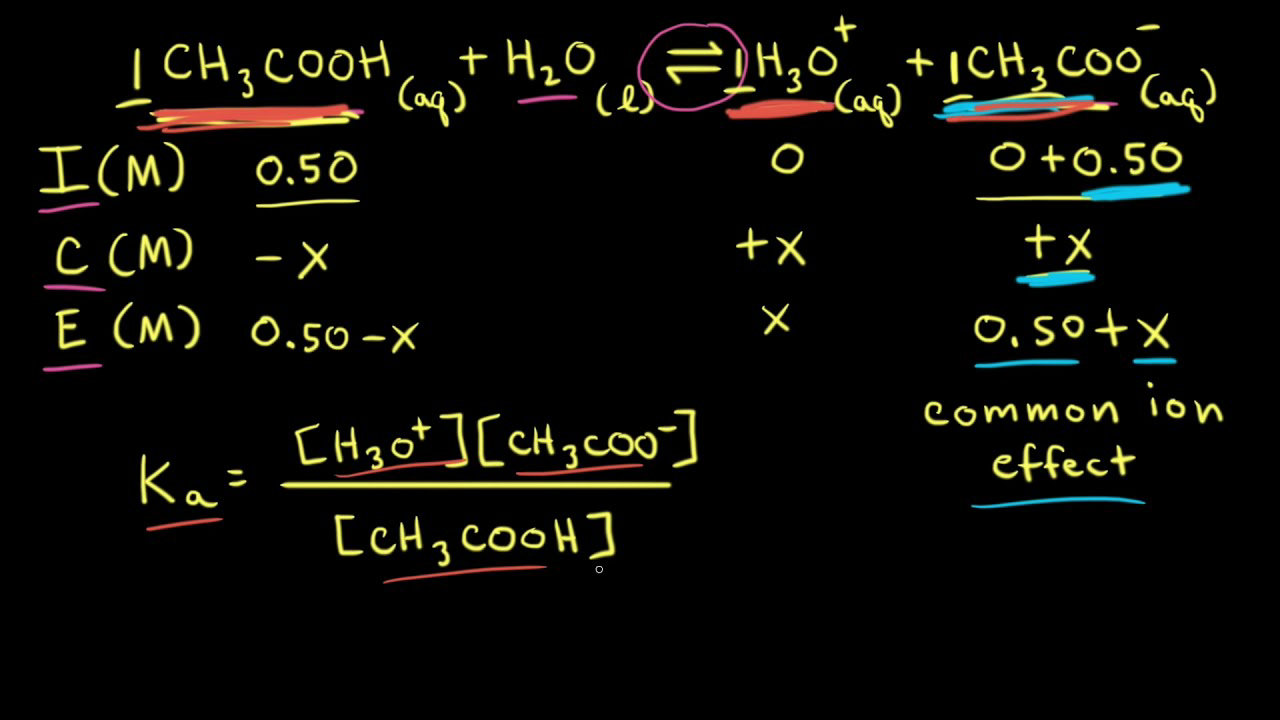
Worked example: Calculating the pH after a weak acid–strong base reaction (excess acid) (video) | Khan Academy
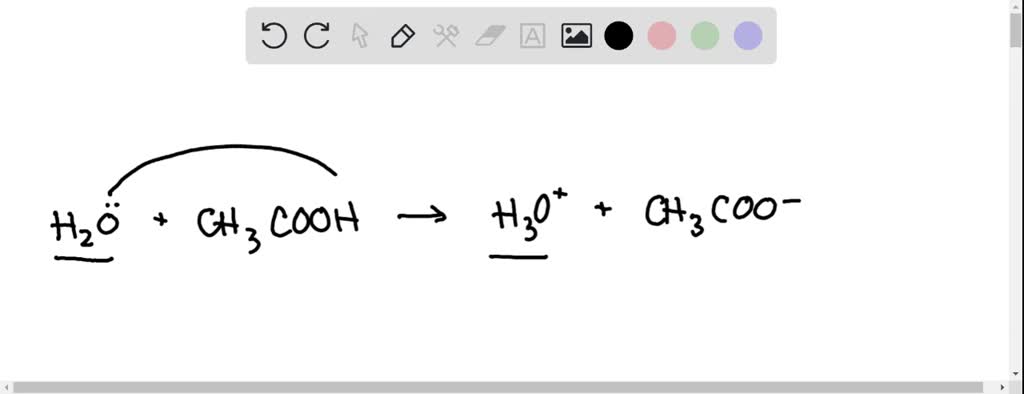
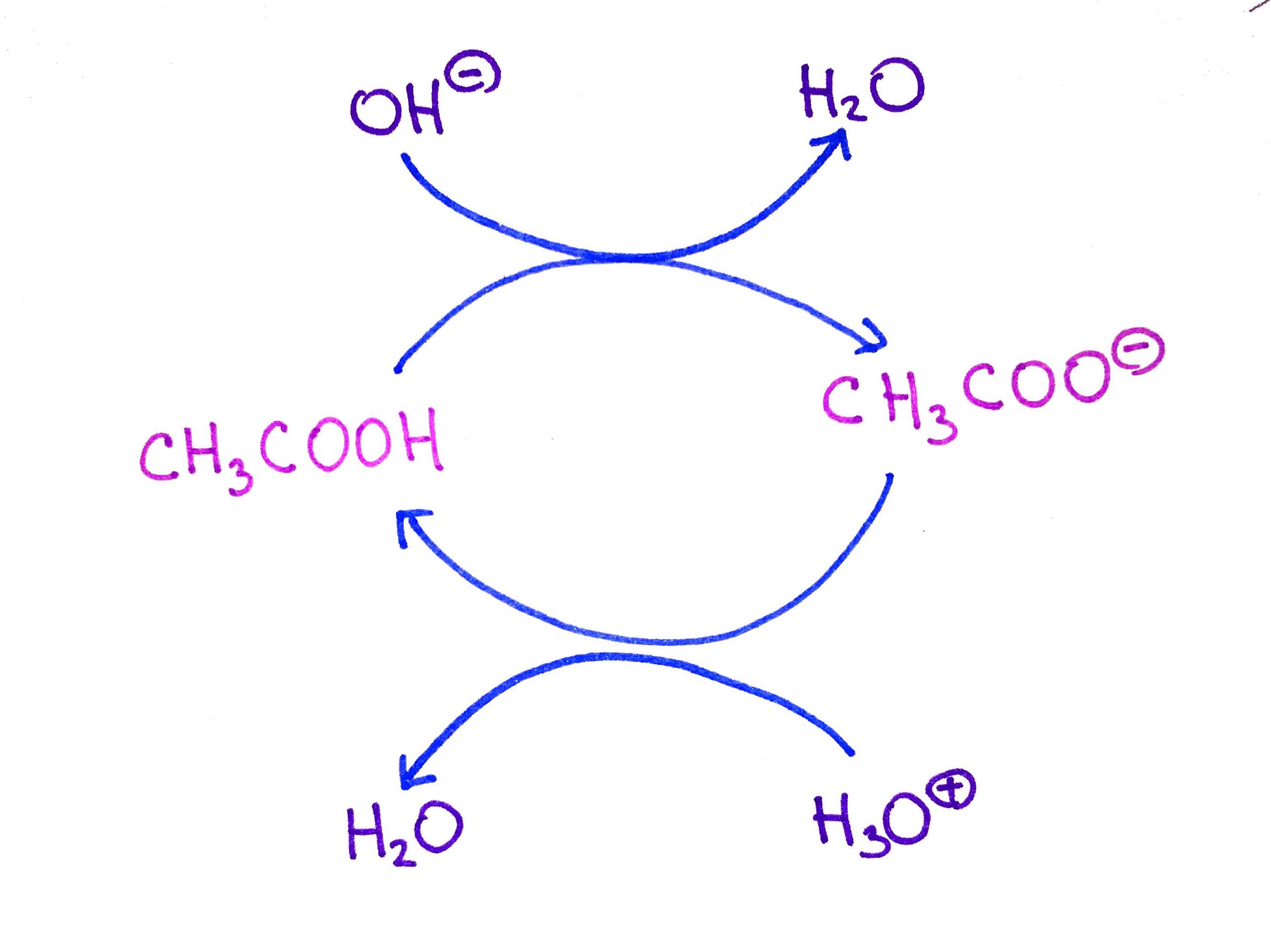
SOLVED: In the following chemical equation, identify the Bronsted-Lowry acid and the Bronsted-Lowry base H2O + CH3COOH CH3COO− + H3O+